 Hyphema is one of the more challenging sequelae associated with blunt ocular trauma. Clinically, it is defined as any accumulation of blood in the anterior chamber. The term microhyphema is used when the blood is suspended in the chamber but does not form a layered clot. Hyphemas are graded on a scale from 1 to 4, based on the amount of blood occupying the anterior chamber.
Hyphema is one of the more challenging sequelae associated with blunt ocular trauma. Clinically, it is defined as any accumulation of blood in the anterior chamber. The term microhyphema is used when the blood is suspended in the chamber but does not form a layered clot. Hyphemas are graded on a scale from 1 to 4, based on the amount of blood occupying the anterior chamber.
A Threat to Vision
Hyphema poses a threat to vision for several reasons:
Visual Compromise. Initially, a large hyphema can obscure the visual axis; in such cases, visual acuity is often severely diminished. As the hemorrhage persists, endothelial blood staining may ensue, which can cause irreparable visual compromise. Studies have shown that the likelihood of corneal blood staining is higher in those with concurrently elevated intraocular pressure (IOP).1
IOP elevation. IOP elevation is considered the most common and significant risk factor for vision loss associated with hyphema, as nearly one-third of afflicted patients have this complication.1 Typically, IOP elevation results from trabecular blockage by erythrocytes, fibrin and associated inflammatory cells. In rare instances, a large clot may prohibit aqueous egress from the posterior chamber, resulting in pupillary block with an attendant, abrupt rise in IOP. While larger hyphemas carry a greater risk of secondary glaucoma, any amount of blood in the anterior chamber can be associated with IOP elevation.2
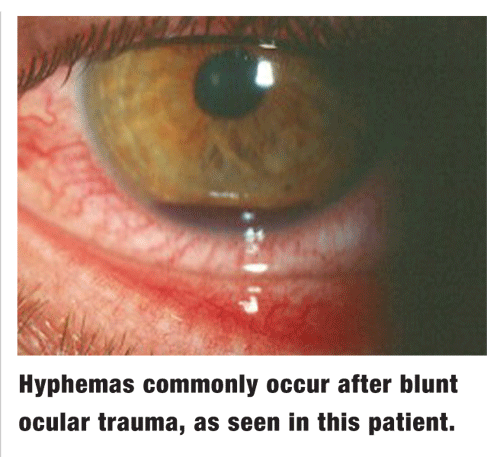 Rebleed. The final concern in cases of hyphema is exacerbation during the resolution period, a phenomenon known as secondary hemorrhage, or rebleed. This is believed to occur from clot lysis and contraction at the site of the initially traumatized vessel. Additional ocular trauma, excessive movement and clotting disorders amplify this risk.
Rebleed. The final concern in cases of hyphema is exacerbation during the resolution period, a phenomenon known as secondary hemorrhage, or rebleed. This is believed to occur from clot lysis and contraction at the site of the initially traumatized vessel. Additional ocular trauma, excessive movement and clotting disorders amplify this risk.
Secondary hemorrhage may occur in as many as 38% of individuals with hyphema and can be even more common in patients of African descent.3,4 The risks of corneal blood staining, IOP elevation and subsequent vision loss are all heightened in cases of secondary hemorrhage.
Management Strategies
Once the potential sequelae are understood, the goals of hyphema management are straightforward: Stabilize the eye to prevent rebleed and facilitate blood resorption, reduce any associated inflammation, and normalize the IOP.
Perhaps the most important therapeutic consideration in achieving these goals is potent cycloplegia. We tend to use atropine 1% b.i.d. for most cases. By immobilizing the iris and ciliary body with atropine, additional trauma to the damaged blood vessels in the angle is prevented. Cycloplegic agents also help to stabilize the blood-aqueous barrier, preventing further leakage of inflammatory cells and proteins. Finally, dilation diminishes the risk of pupillary block or synechia secondary to hyphema.
Cycloplegia was not always considered the standard of care for hyphema management, though. In fact, many of us were taught that miotics (e.g., pilocarpine) were a better therapeutic option. Proponents of pilocarpine claimed that the drug increased the surface area of the iris, allowing for greater, faster absorption of blood; immobilized the iris, preventing additional trauma and diminishing the likelihood of a rebleed; and opened the angle, thereby increasing aqueous outflow and lowering IOP.5
In a study evaluating medical management of hyphema, subjects resolved faster and more completely in the group using pilocarpine 4% compared with the group using atropine 1%. But, the pilocarpine group also had a higher complication rate, displaying greater frequency of secondary hemorrhage, uveitis and elevated IOP.5 Today, we recognize that miotics are a poor choice for managing hyphema. They compromise the blood-aqueous barrier, enhancing leakage of cells and protein into the anterior chamber and increasing the risk of negative sequelae.
Corticosteroids are also typically prescribed in cases of hyphema. They not only address concurrent inflammation, but also reduce the incidence of secondary hemorrhage. By stabilizing the blood-aqueous barrier, corticosteroids diminish the release of proteins, such as plasminogen, a potent mediator of fibrinolysis.6 We typically prescribe prednisolone acetate 1% q.i.d. to q2h, depending on the individual case. Corticosteroids should not be withheld for fear of inducing IOP elevation; it generally takes at least four weeks of high-dose therapy to significantly increase IOP in susceptible individuals.7,8
Ocular hypotensive agents may be indicated in cases with substantially elevated IOP. In general, these are reserved for those with pressures of 26mm Hg or higher.
Assuming no systemic contraindications, any of the following topical agents may be used: beta-blockers (e.g., timolol 0.5%), alpha-adrenergic agonists (e.g., brimonidine 0.1%) or carbonic anhydrase inhibitors (e.g., brinzolamide 1%). The use of prostaglandin analogs (e.g., latanoprost) is discouraged; these drugs are often ineffective in the presence of ocular inflammation.

Controversial Medications
The use of antifibrinolytic agents, such as aminocaproic acid (Amicar, Xanodyne Pharmaceuticals) and tranexamic acid (Cyklokapron, Pfizer), to manage hyphema is controversial. These agents inhibit the conversion of plasminogen to plasmin, impeding fibrin clot degradation. In hyphema, they retard breakdown of coagulated blood and reduce the likelihood of a rebleed.
Some studies have shown measurable benefit with the use of systemic antifibrinolytics, but others were inconclusive.3,9-11 In addition, antifibrinolytics have some undesirable side effectsparticularly vomiting, diarrhea and postural hypotension. They can also promote thromboemboli formation and precipitate renal failure in patients with hemophilia.12 Today, these drugs are generally reserved for severe cases of hyphema in patients at greatest risk for rebleed.
Recently, a topical formulation of aminocaproic acid (Caprogel, ISTA) has been studied as an alternative to systemic antifibrinolytics. Although several small series have demonstrated a reduced rate of secondary hemorrhage with this formulation, it has not yet been approved for use in the United States.13,14
Caution patients to avoid ingesting any substances that can hinder clotting, such as aspirin, ibuprofen, vitamin E and ginko biloba.
Finally, keep in mind that individuals with hemoglobinopathiesparticularly sickling disordersare at greatest risk for complications.16 Obtain a careful medical history on all patients, especially those of African descent.
1. Read J. Traumatic hyphema: surgical vs. medical management. Ann Ophthalmol 1975 May;7(5):659-62,664-6,668-70.
2. Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of traumatic hyphema. Surv Ophthalmol 2002 Jul-Aug;47(4):297-334.
3. Volpe NJ,
4. Spoor TC, Kwitko GM, O"Grady JM, Ramocki JM. Traumatic hyphema in an urban population. Am J Ophthalmol 1990 Jan 15;109(1):23-7.
5. Chaddah MR, Gupta SP. Management of traumatic hyphema. Indian J Ophthalmol 1978 Jul;26(2):32-4.
6. Ng CS, Strong NP, Sparrow JM, Rosenthal AR. Factors related to the incidence of secondary hemorrhage in 462 patients with traumatic hyphema. Eye 1992;6(Pt 3):308-12.
7. Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol 1965 Apr;4:198-205.
8. Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol 2006 Apr;17(2):163-7.
9. Kutner B, Fourman S, Brein K, et al. Aminocaproic acid reduces the risk of secondary hemorrhage in patients with traumatic hyphema. Arch Ophthalmol 1987 Feb;105(2):206-8.
10. McGetrick JJ, Jampol LM, Goldberg MF, et al. Aminocaproic acid decreases secondary hemorrhage after traumatic hyphema. Arch Ophthalmol 1983 Jul;101(7):1031-3.
11. Teboul BK, Jacob JL, Barsoum-Homsy M, et al. Clinical evaluation of aminocaproic acid for managing traumatic hyphema in children. Ophthalmology 1995 Nov;102(11):1646-53.
12. Pitts TO, Spero JA, Bontempo FA, Greenberg A. Acute renal failure due to high-grade obstruction following therapy with epsilon-aminocaproic acid. Am J Kidney Dis 1986 Dec;8(6):441-4.
13. Crouch ER Jr, Williams PB, Gray MK, et al. Topical aminocaproic acid in the treatment of traumatic hyphema. Arch Ophthalmol 1997 Sep;115(9):1106-12.
14. Pieramici DJ, Goldberg MF, Melia M, et al. A phase III, multicenter, randomized, placebo-controlled clinical trial of topical aminocaproic acid (Caprogel) in the management of traumatic hyphema. Ophthalmology 2003 Nov;110(11):2106-12.
15. Recchia FM, Saluja RK, Hammel K, Jeffers JB. Outpatient management of traumatic microhyphema. Ophthalmology 2002 Aug;109(8):1465-70.
16. Nasrullah A,

