  |
The sacred motto in medicine is, “Above all else, do no harm.” This seems reasonable enough, but sometimes there are untoward effects of therapy. For example, we all know that topical steroids can cause glaucoma and cataracts. But several other frequently used agents can yield adverse effects, too. In this month’s column, we’ll review of few of these.
Avastin
In recent years, intravitreal Avastin (bevacizumab, Genentech) has been used regularly to treat neovascular disease and macular edema resulting from diabetic retinopathy, retinal vein occlusion, and several other ocular conditions that cause retinal capillary closure and hypoxia. These conditions all feature up-regulation of vascular endothelial growth factor (VEGF), which stimulates angiogenesis and promotes endothelial cell migration and proliferation.1 Also, VEGF is associated with blood-retinal barrier breakdown, increased vascular permeability and fluid leakage. These mechanisms precipitate neovascularization and retinal edema.2
Avastin, a VEGF inhibitor, is a humanized monoclonal antibody that was initially developed as treatment for metastatic colorectal cancer.3 Such inhibitors block the up-regulation of VEGF, eliminate the stimulus for neovascularization and reduce the vascular permeability that causes retinal edema.
One of the potential complications of Avastin use is endophthalmitis associated with intraocular injection.4 While uncommon (occurring in approximately one in 3,000 injections), the threat of potential vision loss is tremendous. Use of an eyelid speculum and povidone iodine, as well as avoidance of needle contact with the eyelid margin, greatly reduce the patient’s risk of post-injection endophthalmitis.
Despite these risk reduction strategies, vision loss from endophthalmitis cannot be absolutely eliminated. Intravitreal Avastin typically is prepared by a compounding pharmacy, which naturally introduces a risk of contamination. As national media sources reported throughout the country, 12 patients in South Florida developed endophthalmitis in July 2011 after receiving intravitreal Avastin injections.5,6 Seven of the 12 patients ultimately were enucleated, and only one patient achieved a final visual acuity better than finger counting.
Because this situation included an unusually high number of endophthalmitis cases within a short time period in one geographic area, some researchers speculated that the cause originated from one source. Ultimately, this proved to be the case, as Streptococcus mitis/oralis was cultured from the majority of patients and from all unused syringes that came from a single compounding pharmacy.7 While much of the country reacted in fear, this was a localized issue that was caused by a lack of infection protocol at one facility. Overall, however, intravitreal treatment with Avastin is a safe, effective option for patients.
Jetrea
Vitreomacular adhesion and vitreomacular traction syndrome may reduce vision and can progress to macular hole development, which can cause even greater visual morbidity. Vitreoretinal adhesion and traction exist due to fibrocellular proliferation at the macula. Previously, these findings only could be relieved through vitrectomy. Now, the proteolytic enzyme ocriplasmin (Jetrea, ThromboGenics) can resolve vitreomacular adhesions via intravitreal injection rather than invasive surgery. Ocriplasmin cleaves fibronectin and laminin at the vitreoretinal interface. Success has been reported in up to 25% of patients with symptomatic vitreomacular traction syndrome following a single intravitreal injection of Jetrea.8,9
Recently, however, there have been reports of possible retinal toxicity and vision loss after Jetrea use.10 One report detailed a 71-year-old woman with symptomatic vitreomacular traction who received intravitreal Jetrea and experienced vision darkening in dim illumination that persisted for four months, despite traction release and a general improvement in visual acuity. Spectral-domain optical coherence tomography (SD-OCT) showed a disruption of her photoreceptor inner segment/outer segment layer. Additionally, a reduction of electroretinogram (ERG) amplitudes accounted for her complaint of darkened vision. The authors speculated that Jetrea may have a diffuse enzymatic effect on the photoreceptors or the retinal pigment epithelium located outside of immediate areas of vitreomacular adhesion.10
|
|
|
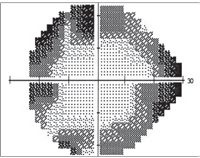
Patients who use the antiepileptic agent Sabril may experience peripheral vision field constriction, as seen here.
|
Another report detailed a 63-year-old woman who experienced acute panretinal dysfunction after intravitreous Jetrea. The patient demonstrated acute visual acuity loss, visual field constriction, attenuated retinal arteries, loss of retinal outer segment retinal signals on SD-OCT and severely reduced ERG responses. The authors concluded that retinal dysfunction associated with intravitreous Jetrea injection is not limited to the macular region and may involve the entire retina. Further, they suggested that enzymatic cleavage of intraretinal laminin is a biologically plausible mechanism for acute ocriplasmin retinal toxic effects.11
While these reports may cause concern and perhaps hesitation to recommend Jetrea as an alternative to vitreoretinal surgery, be aware that the medication otherwise showed a positive safety profile prior to these reports.
Jetrea is supplied in a single-use glass vial, which contains 0.5mg ocriplasmin for intravitreal injection after dilution. Thus, Jetrea has to be reconstituted in the office. While the toxic effects recently reported could be due to the formulation itself, some have speculated that such complications may have arisen due to improper dilution or other untoward effects from the reconstitution process.
Sabril
Sabril (vigabatrin, Lundbeck) is an effective antiepileptic drug used to treat refractory complex partial seizures and infantile spasms. While Sabril has been used in other countries since 1989, it didn’t receive FDA approval until 2009. This 20-year delay likely was influenced by the drug’s potential to cause profound visual field constriction. Specifically, Sabril has been noted to cause reduction of cone d-wave amplitude on ERG testing.12
Binasal defects are detected initially, progressing to bilateral concentric field constriction that preserves central acuity.13 In one prospective analysis of 734 patients treated with Sabril, 71% exhibited visual field defects.14 A retrospective analysis documented visual field defects in 66% of men and 54% of women who used Sabril.15
Further, researchers noted that the prevalence of visual field loss seems to increase with dose size, patient age and duration of therapy.16,17 Beyond visual field loss, another study indicated that the parapapillary retinal nerve fiber layer is notably thin on SD-OCT scans of patients who used Sabril.18
Because Sabril is not a commonly prescribed drug, we wanted to alert eye care practitioners of the possibility of potentially devastating visual field loss and the need for ongoing screening. It appears that visual field loss does not progress after Sabril cessation, but functional visual field improvement won’t occur either.
For this reason, order threshold perimetry at least every six months for patients on Sabril treatment. Also, consider SD-OCT as an adjunctive monitoring tool, particularly in children who cannot perform perimetry. In these instances, ERG may also be a helpful. Should any structural or functional dysfunction develop, the medication most likely will need to be
discontinued immediately upon consult with patients’ neurologists.
Any medical treatment always carries some risk of adverse effects––some anticipated, some not. Occasionally, there may be quality issues associated with otherwise safe treatments. Additionally, there may be systemic medications with profound ocular implications that are unfamiliar to many practitioners.
1. Priglinger SG, Wolf AH, Kreutzer TC, et al. Intravitreal bevacizumab injections for treatment of central retinal vein occlusion: six-month results of a prospective trial. Retina. 2007 Oct;27(8):1004-12.
2. Pe’er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998 Mar;105(3):412-6.
3. Marshall J. The role of bevacizumab as first-line therapy for colon cancer. Semin Oncol. 2005 Dec;32(6 Suppl 9):S43-7.
4. Schwartz SG, Flynn HW Jr. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor injections. Curr Ophthalmol Rep. 2014;2(1):1-5.
5. Goldberg RA. Streptococcus endophthalmitis outbreak after intravitreal injection of bevacizumab: one-year outcomes and investigative results. Ophthalmology. 2013;120(7):1448-53.
6. Goldberg RA, Flynn HW Jr, Isom RF, et al. An outbreak of Streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012 Feb;153(2):204-8.
7. Matthews JL, Dubovy SR, Goldberg RA, Flynn HW Jr. Histopathology of Streptococcus mitis/oralis endophthalmitis after intravitreal injection with bevacizumab: a report of 7 patients. Ophthalmology. 2014;121(3):702-8.
8. Kuppermann BD. Ocriplasmin for pharmacologic vitreolysis. Retina. 2012;32 Suppl 2:S225-8.
9. Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367(7):606-15.
10. Tibbetts MD. Vision loss after intravitreal ocriplasmin: correlation of spectral-domain optical coherence tomography and electroretinography. JAMA Ophthalmol. 2014;132(4):487-90.
11. Fahim AT, Khan NW, Johnson MW. Acute panretinal structural and functional abnormalities after intravitreous ocriplasmin injection. JAMA Ophthalmol. 2014 Apr 1;132(4):484-6.
12. Dragas R, Westall C, Wright T. Changes in the ERG d-wave with vigabatrin treatment in a pediatric cohort. Doc Ophthalmol. 2014 Jul 10. [Epub ahead of print].
13. Willmore LJ, Abelson MB, Ben-Menachem E, et al. Vigabatrin: 2008 update. Epilepsia. 2009;50(2):163-73.
14. Wild JM, Chiron C, Ahn H, et al. Visual field loss in patients with refractory partial epilepsy treated with vigabatrin: final results from an open-label, observational, multicentre study. CNS Drugs. 2009;23(11):965-82.
15. Wild JM, Fone DL, Aljarudi S, et al. Modelling the risk of visual field loss arising from long-term exposure to the antiepileptic drug vigabatrin: a cross-sectional approach. CNS Drugs. 2013;27(10):841-9.
16. Maguire MJ, Hemming K, Wild JM, et al. Prevalence of visual field loss following exposure to vigabatrin therapy: a systematic review. Epilepsia. 2010;51(12):2423-31.
17. Pellock JM. Balancing clinical benefits of vigabatrin with its associated risk of vision loss. Acta Neurol Scand Suppl. 2011;(192):83-91.
18. Clayton LM, Devile M, Punte T, et al. Patterns of peripapillary retinal nerve fiber layer thinning in vigabatrin-exposed individuals. Ophthalmology. 2012;119(10):2152-60.

