 |
The patient was diagnosed with bilateral primary chronic angle closure glaucoma (PCACG). The therapeutic goals were to change the anatomic status of his closed angles and lower his IOP. Angle closure glaucoma in any form is far less common than open angle glaucoma, making management sometimes confusing. Additionally, new theories are challenging the way we address this condition. This month, we will review the management of patients with chronic angle closure glaucoma.
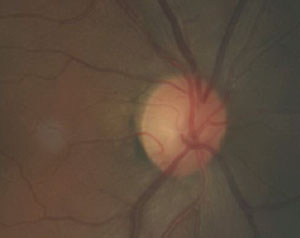 | |
| This fundus image shows a patient with primary chronic angle closure glaucoma. |
PCACG Patients
Patients with PCACG are typically older and asymptomatic, and women are more commonly affected than men.1 Biomicroscopically, there will be a shallow anterior chamber, though typically deeper than in primary acute angle closure glaucoma, where iris bombé is characteristically seen. The anterior chamber angle may be appositionally closed and opened upon manual pressure using a four-mirror gonio lens, or the angle may be closed with broad areas of PAS. The superior and temporal quadrants of the anterior angle may be the earliest sites of the synechial angle closure, with gradual extension on the nasal quadrant, until the angle closes at the inferior quadrant.2 While in most cases there is asymmetric closure first involving the superior angle, there can be an even, circumferential process that slowly progresses to symmetrical closure. This is called “creeping angle closure” and appears as an angle that becomes progressively more shallow over time.3
Symptoms
Anatomical features act in concert to cause shallowing of the anterior chamber. As a patient ages, thickening of the crystalline lens leads to a relative pupil block, putting the iris into apposition with the trabecular meshwork. Because the closure is slow, symptoms you would typically see with acute angle closure, such as pain, nausea and vision loss, are absent and patients remain unaware of the elevating IOP.4,5
Treatments
Conventional thinking has long held that all cases of primary angle closure resulting from relative pupil block need to undergo laser peripheral iridotomy (LPI) as soon as possible after diagnosis. This allows aqueous flow from the posterior chamber to the anterior chamber to bypass any pupil blockage. This can stimulate the backward relaxation of the iris, with a resultant deepening of the chamber and opening of the angle.6,7
However, a significant number of these PCACG eyes will manifest residual angle closure even after LPI.6 Additionally, there often will be elevated IOP due to damage to the trabecular meshwork from appositional and synechial closure.8,9 Most eyes with PCACG require further treatment to control IOP, including trabeculectomy and medical therapy.10
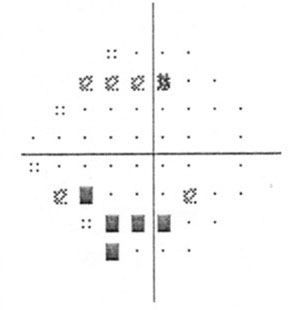 | |
| Visual field damage in a patient with primary chronic angle closure glaucoma. |
Pharmaceuticals
Medical therapy that has been successful in reducing IOP in eyes with PCACG include beta blockers, miotics, alpha-2 adrenergic agonists, and prostaglandin analogs.11,12 Research shows that the prostaglandins work especially well in eyes with PCACG that need IOP reduction both before and after LPI, though the mechanism remains unclear.13-16
Lensectomy
Because the crystalline lens can contribute to the development of PCACG, lensectomy remains a viable option for some eyes. The exact mechanism by which cataract surgery lowers IOP in glaucomatous eyes remains unknown. Evidence suggests that in eyes with narrow and closed angles, the level of IOP lowering after cataract surgery is proportional to the resultant widening of the angle.17 Phacoemulsification and intraocular lens implantation can lower IOP, reduce or remove the critical anatomical characteristics that produce pupillary block, and subsequently increase angle width.17 Research shows, in eyes with PCACG and co-existing cataract, that phacoemulsification alone can significantly reduce both IOP and the need for topical therapy, supporting cataract removal as a primary treatment rather than LPI and stepped-medical therapy.18,19
The role of clear lensectomy (i.e., extraction of the non-cataractous lens) in patients with PCACG is unclear. The Effectiveness in Angle Closure Glaucoma of Lens Extraction (EAGLE) study, a prospective, randomized clinical trial now underway, will compare the safety and effectiveness of LPI and medical therapy to clear lens extraction for patients with newly diagnosed PCACG.20 With any luck, the results of the EAGLE study will help guide management of these challenging patients.
We educated our patient about his condition and the ultimate need for surgical intervention. Because his lenses were relatively clear with poor visual potential in his left eye (and perhaps partially due to his lack of insurance) he opted to initially undergo a less invasive and less expensive LPI. After the procedure, his anterior chamber angles opened to at least posterior pigmented trabecular meshwork (with minimal PAS) with an immediate reduction in IOP. However, in the course of several months, his IOP did elevate due to trabecular compromise induced by the appositional closure, necessitating use of a topical glaucoma medication.
While this patient was successfully managed with conventional methods, the EAGLE study may ultimately change our therapeutic algorithm from LPI and medical therapy to clear lens extraction.
1. Bonomi L, Marchini G, Marraffa M, et al. Epidemiology of angle-closure glaucoma: prevalence, clinical types, and association with peripheral anterior chamber depth in the Egna-Neumarket Glaucoma Study. Ophthalmology. 2000;107(5):998-1003.2. Mok K, Lee V. Synechial angle closure pattern in Chinese chronic primary angle-closure glaucoma patients. J Glaucoma. 2001;10(5):427-8.
3. Lowe RF. Primary creeping angle closure glaucoma. Br J Ophthalmol 1964;48:544.
4. Wang T, Liu L, Li Z, et al. Studies of mechanism of primary angle closure glaucoma using ultrasound biomicroscope. Zhonghua Yan Ke Za Zhi. 1998;34(5):365-8.
5. Tarongoy P, Ho CL, Walton DS. Angle-closure glaucoma: the role of the lens in the pathogenesis, prevention, and treatment. Surv Ophthalmol. 2009;54(2):211-25.
6. He M, Friedman DS, Ge J, et al. Laser peripheral iridotomy in primary angle-closure suspects: biometric and gonioscopic outcomes: the Liwan Eye Study. Ophthalmology. 2007;114(3):494-500.
7. Hsiao CH, Hsu CT, Shen SC, et al. Mid-term follow-up of Nd:YAG laser iridotomy in Asian eyes. Ophthalmic Surg Lasers Imaging. 2003;34(4):291-8.
8. Chen MJ, Cheng CY, Chou CK, et al. The long-term effect of Nd:YAG laser iridotomy on intraocular pressure in Taiwanese eyes with primary angle-closure glaucoma. J Chin Med Assoc. 2008;71(6):300-4.
9. Sihota R, Lakshmaiah NC, Walia KB, et al. The trabecular meshwork in acute and chronic angle closure glaucoma. Indian J Ophthalmol. 2001;49(4):255-9.
10. Rosman M, Aung T, Ang LP, et al. Chronic angle-closure with glaucomatous damage: long-term clinical course in a North American population and comparison with an Asian population. Ophthalmology. 2002;109(12):2227-31.
11. Ruangvaravate N, Kitnarong N, Metheetrairut A, et al. Efficacy of brimonidine 0.2 per cent as adjunctive therapy to beta-blockers: a comparative study between POAG and CACG in Asian eyes. J Med Assoc Thai. 2002;85(8):894-900.
12. Aung T, Wong HT, Yip CC, et al. Comparison of the intraocular pressure-lowering effect of latanoprost and timolol in patients with chronic angle closure glaucoma: a preliminary study. Ophthalmology. 2000;107(6):1178-83.
13. How AC, Kumar RS, Chen YM, et al. A randomised crossover study comparing bimatoprost and latanoprost in subjects with primary angle closure glaucoma. Br J Ophthalmol. 2009;93(6):782-6.
14. Chen MJ, Chen YC, Chou CK, et al. Comparison of the effects of latanoprost and bimatoprost on intraocular pressure in chronic angle-closure glaucoma. J Ocul Pharmacol Ther. 2007;23(6):559-66.
15. Chen MJ, Chen YC, Chou CK, et al. Comparison of the effects of latanoprost and travoprost on intraocular pressure in chronic angle-closure glaucoma. J Ocul Pharmacol Ther. 2006;22(6):449-54.
16. Gupta V, Srinivasan G, Sharma A, et al. Comparative evaluation of bimatoprost monotherapy in primary chronic angle closure and primary open angle glaucoma eyes: a three-year study. J Ocul Pharmacol Ther. 2007;23(4):351-8.
17. Pachimkul P, Intajak Y. Effect of lens extraction on primary angle closure in a Thai population. J Med Assoc Thai. 2008;91(3):303-8.
18. Lai JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction by phacoemulsification in eyes with primary angle-closure glaucoma (PACG) and co-existing cataract: a prospective case series. J Glaucoma. 2006;15(1):47-52.
19. Hata H, Yamane S, Hata S, et al. Preliminary outcomes of primary phacoemulsification plus intraocular lens implantation for primary angle-closure glaucoma. J Med Invest. 2008;55(3-4):287-91.
20. Azuara-Blanco A, Burr JM, Cochran C, et al. Effectiveness in Angle-closure Glaucoma of Lens Extraction (EAGLE) Study Group. The effectiveness of early lens extraction with intraocular lens implantation for the treatment of primary angle-closure glaucoma (EAGLE): study protocol for a randomized controlled trial. Trials. 2011;12:133.

