We’ve often wondered why so many antibiotic eye drops are prescribed. Our best guess is that when clinicians are presented with red eyes, and they’re uncertain of the diagnosis, they default to an innocuous therapeutic trial. After all, antibiotics, by and large, are certainly safe, but they render no therapeutic effect unless there are proliferating pathological bacteria on the ocular surface. Thankfully, the natural history of many external eye diseases are rapidly self-limiting, and so there is at least the appearance of a therapeutic effect.
Speaking of placebo power, an article by Marc Abelson, M.D., et al, in the June 2008 American Journal of Ophthalmology, found that when comparing topical azithromycin to vehicle, the clinical cure rate for gram-negative bacteria was 91.4% for the drug and 78.6% for the vehicle.1 Against gram-positive bacteria, the clinical cure rate for the drug was 89.4% and 60.6% for the vehicle. Now that’s a rather remarkable cure rate for a mere vehicle!
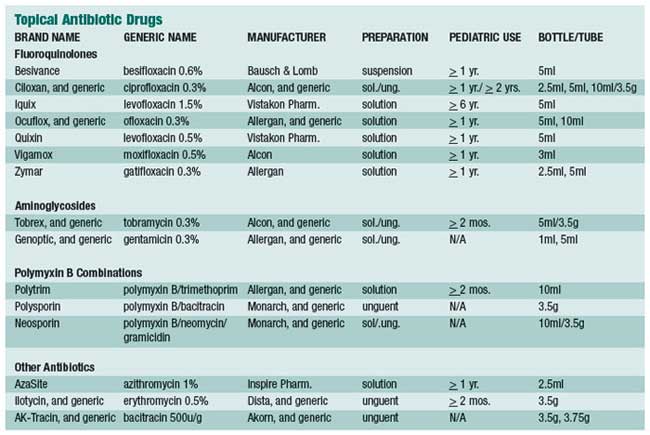
The summary statement from this article succinctly concludes: “Because it [azithromycin] was well tolerated in this population, it may be a viable treatment option for children and adults with bacterial conjunctivitis.” We certainly agree. AzaSite (azithromycin 1%, Inspire Pharmaceuticals) is formulated with DuraSite (a vehicle owned by InSite Vision, Inc.), a new co-adhesive matrix that holds active drug at the ocular surface for long periods of time (a few hours), thereby yielding good efficacy with much less frequent dosing. Indeed, the recommended dose schedule for AzaSite is one drop twice a day (about eight to twelve hours apart) for two days, then only once daily for five more days. This dosing schedule can be especially helpful when treating young children.
There is considerable discussion of the use of AzaSite in helping control posterior blepharitis/meibomian gland dysfunction. Some advocate the use of a drop of AzaSite at bedtime with gently rubbing of the excess medicine along the eyelid margins. Others advocate the use of a drop on the closed eyelids and then rub the medicine along the eyelid margins. Whether one should do either of these once or twice daily, and for how long, is not yet established. Our thought is to try the former approach twice a day for a week, then just at bedtime for two more weeks. This is completely anecdotal, as no large, prospective, double-blind studies provide any scientific guidance. Furthermore, we wonder if a month or two or oral doxycycline at 50mg per day with or without a twoweek course of antibiotic steroid eye drops four times a day would be more, or less, effective. With the best interest of the patient being foremost, we, as a professional community, should try a number of clinical approaches with the hope of establishing a consensus of expert opinion as to the most effective approach for posterior blepharitis.

The big concern in treating bacterial infections is the continuing emergence of resistant bacterial species, particularly methicillin resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE). Now this concern plagues the primary care community more than the eye care community, mainly because eye doctors are privileged to treat eye infections with topical eye drops, which are usually able to overpower the most stubborn bacteria. This is because such high concentrations of drug can be achieved on the ocular surface with topical application, as opposed to a small pill diluted in about five liters of blood. It is not a fair fight. We may share more of the burden with the primary care community when it comes to treating eyelid infections, where orally administered antibiotics are employed.
Although the standard in vitro susceptibility testing is important in the management of infectious diseases, MIC values should be interpreted in light of the expected delivery and drug concentration at the site of infection. This point may be especially important for ocular infections treated topically, where local concentrations of antibiotic may be much higher than maximum serum levels obtained from systemic dosing.
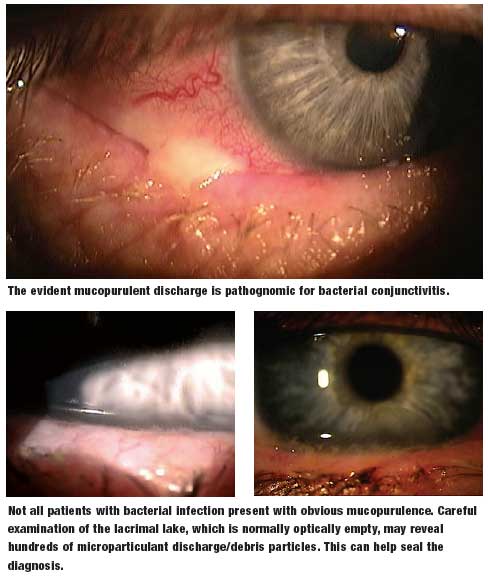
To give more insight into microbial resistance and antibiotic effectiveness, a nationwide system of study and evaluation of these concerns was established in 1996, the year levofloxacin was brought to market. This system is called “Tracking Resistance in the United States Today,” or TRUST. More recently, in 2005 to 2006, Ocular TRUST was established, which looks specifically at ocular bacterial isolates.2 You’ll be rather amazed at what has been discovered. Basically, with regard to MRSA infections, the fluoroquinolones—levofloxacin, moxifloxacin and gatifloxacin—all performed identically and were effective only about 20% of the time. In contrast, trimethoprim was effective against 95% of MRSA isolates. These fluoroquinolones were effective against 80% of methicillinsensitive Staphylococcus aureus (MSSA) isolates, whereas both tobramycin and trimethoprim were approximately 95% effective.
Regarding Streptococcus pneumoniae, these three fluoroquinolones were 100% effective. Against Haemophilus influenzae, the three fluoroquinolones were 100% effective and trimethoprim was about 85% effective.
Now, from a clinical practical perspective, what does all this mean? To explain, the Ocular TRUST authors state: “Although in vitro activity may be predictive of efficacy, it is not a guarantee because a multitude of factors influence clinical response.”2 Most all of our currently available topical antibiotics, used frequently enough, will eradicate most bacterial infections of the conjunctiva and cornea. If you’re not achieving clinical cure with a fluoroquinolone, an aminoglycoside or trimethoprim, then switch or add one of these other classes/drugs. On rare occasions, we add Polysporin or Neosporin ophthalmic ointment at bedtime.
The general principle of treating with antibiotics or a corticosteroid is to have the patient use whichever drug you prescribe frequently (for example, every two hours) for at least a couple of days before dropping down to q.i.d. for four to six more days. It is not particularly the antibiotic chosen, but the frequency of the instillation that determines the clinical efficacy of most drugs.
There are two main subdivisions of antibiotics:
- Bactericidal and bacteriostatic. Bactericidal antibiotics are represented by the aminoglycosides, the fluoroquinolones, the penicillins, and the cephalosporins. Conversely, bacteriostatic drugs are sodium sulfacetamide, trimethoprim, and to some degree, erythromycin.
- Concentration-dependent and time-dependent. Concentrationdependent drugs are aminoglycosides and fluoroquinolones. Timedependent drugs are the macrolides (erythromycin, azithromycin, and clarithromycin), and the penicillins.
 All ophthalmic antibiotics with
widely used oral counterparts will
ultimately develop resistance—and
yes, that includes the so-called
“fourth generation” varieties. It is
evident that gram-positive bacterial pathogens (particularly Staphylococcus
aureus and epidermidis) are
the most common causes of ocular
infections, but a sizable minority is
gram-negative (mostly Serratia,
Pseudomonas and Moraxella
species). With this in mind, we need
to use broad-spectrum drugs.
All ophthalmic antibiotics with
widely used oral counterparts will
ultimately develop resistance—and
yes, that includes the so-called
“fourth generation” varieties. It is
evident that gram-positive bacterial pathogens (particularly Staphylococcus
aureus and epidermidis) are
the most common causes of ocular
infections, but a sizable minority is
gram-negative (mostly Serratia,
Pseudomonas and Moraxella
species). With this in mind, we need
to use broad-spectrum drugs.
Fortunately, nearly all topical ophthalmic antibiotics are broad spectrum, and so meet the needs of the marketplace. Face it: a drug having only gram-positive or only gram-negative activity would require definitive culture results in order to enact a rational therapy for moderate to severe infections, which is, in practicality, irrational.
Now, let’s take a clinically practical look at each drug:
Bacitracin
Developed in 1943, bacitracin
is an excellent
gram-positive bactericidal
drug. Its mechanism
of action is the destruction
of the bacterial cell
wall. It is only available
as an ophthalmic ointment,
which severely
limits its clinical use,
because adults do not
like to have highly viscous
ointments in their
eyes. It has two main
uses: infectious blepharitis and for
nocturnal supplementation to topical
eye drops in the treatment of bacterial corneal ulcer. Bacitracin is
generically available.
bacterial corneal ulcer. Bacitracin is
generically available.
Bacitracin with Polymyxin B
Polymyxin B is excellently bactericidal against most gram-negative bacterial species. Its mechanism of action is destruction of the bacterial cell membrane. Polymyxin B is not a stand-alone drug, however. It is always found in combination products to provide coverage against gram-negative pathogens. The combination with bacitracin is known as Polysporin ophthalmic ointment, and it is not available in the United States in eye drop form. The ointment formulation is available as a generic product. OTC (non-ophthalmic) Polysporin comes as a 15gm tube, contains the same two drugs, and performs identically.
Bacitracin, Polymyxin B and Neomycin
 Neomycin is an aminoglycoside,
which, like polymyxin, is not found
as a stand-alone drug. It is always
found in a combination formulation.
Neomycin works to inhibit
protein synthesis and is inherently
broad spectrum, with the notable
exception of Pseudomonas species
(this is why polymyxin B is commonly
combined within neomycin).
Neomycin is an excellent drug, but
it is mostly known for its potential
to cause a Type IV delayed hypersensitivity
reaction, which is manifested
as a low-grade blepharoconjunctivitis,
with variable expression
of inflammatory blepharodermatitis.
This red, weepy skin
reaction can easily be reversed by
drug cessation. Such so-called
“neomycin reactions” occur in 5 to
10% of treated patients,
and is nothing more
than an inconvenience.
Neomycin is an aminoglycoside,
which, like polymyxin, is not found
as a stand-alone drug. It is always
found in a combination formulation.
Neomycin works to inhibit
protein synthesis and is inherently
broad spectrum, with the notable
exception of Pseudomonas species
(this is why polymyxin B is commonly
combined within neomycin).
Neomycin is an excellent drug, but
it is mostly known for its potential
to cause a Type IV delayed hypersensitivity
reaction, which is manifested
as a low-grade blepharoconjunctivitis,
with variable expression
of inflammatory blepharodermatitis.
This red, weepy skin
reaction can easily be reversed by
drug cessation. Such so-called
“neomycin reactions” occur in 5 to
10% of treated patients,
and is nothing more
than an inconvenience.
This triple antibiotic is an excellent, broad-spectrum drug that is available generically in both solution and ointment form. Because of solubility issues, gramicidin replaces bacitracin in the solution form. Gramicidin and bacitracin are clinical equivalents in combating gram-positive bacteria.
Trimethoprim with Polymyxin B
Trimethoprim is an excellent, broad-spectrum bacteriostatic antibiotic. Though it inhibits bacterial folic acid synthesis in a manner similar to the sulfonamides, it is not a sulfa-related drug. Systemically, trimethoprim combined with sulfamethoxazole, historically marketed as Bactrim (AR Scientific) or Septra (Monarch), is a drug of choice when treating systemic soft tissue infections caused by MRSA pathogens. As can be deduced, trimethoprim is not active against some gram-negative bacteria, which is why it is combined with polymyxin B. Because this combination drug is particularly effective against Streptococcus pneumoniae and Haemophilus influenzae, two common pathogens in the pediatric population, this is the drug of choice in children with bacterial conjunctivitis. Originally known by the brand name Polytrim (Allergan), this ophthalmic solution is now available generically

Erythromycin
The most common use of erythromycin
in eye care is as a nocturnal
lubricant when a lubricant with
antibiotic properties is desired.
Erythromycin, topically and systemically,
has limited use because of its
poor resistance profile. It is hardly
ever used to actively treat an infection,
but is almost always used in a
prophylactic role. Just as with bacitracin,
ophthalmic erythromycin is
available only as an ointment,
which limits its practical application. 
Erythromycin is essentially bacteriostatic against many gram-positive and gram-negative bacteria. It exerts its antibacterial action through the interruption of protein synthesis. However, because of its systemic use for decades, resistance (particularly against Staph. species) has developed and has limited its clinical usefulness.
Azithromycin
This more modern rendition of a macrolide antibiotic is well known by its original brand name of Zithromax (Pfizer). It is prescribed systemically as a Z-Pak, and is available in a packet of six 250mg capsules; Tri-Pak, which is available in a packet of three 500mg capsules; or as a 1,000mg oral suspension and 2,000mg oral suspension (Zmax).
 The ophthalmic
formulation of
azithromycin, known
as AzaSite (Inspire
Pharmaceuticals), is
produced as a highviscosity
eyedrop
solution. Since azithromycin
has a particularly
prolonged
intracellular half-life,
both in systemic and topical form,
it is dosed less frequently than other
ophthalmic drugs. For AzaSite, the
standard dosage is one drop every
eight to 12 hours for the first two
days, then one drop daily for five
more days.
The ophthalmic
formulation of
azithromycin, known
as AzaSite (Inspire
Pharmaceuticals), is
produced as a highviscosity
eyedrop
solution. Since azithromycin
has a particularly
prolonged
intracellular half-life,
both in systemic and topical form,
it is dosed less frequently than other
ophthalmic drugs. For AzaSite, the
standard dosage is one drop every
eight to 12 hours for the first two
days, then one drop daily for five
more days.
Its mechanism of action is the inhibition of protein synthesis. Because of its spectrum of activity, it, like trimethoprim (with polymyxin B), has its greatest value in treating pediatric bacterial eye infections. Its main advantage is its more patient-friendly dosing frequency. Since it is only available by brand name, it is relatively more expensive than generic Polytrim.
AzaSite comes in a white, opaque bottle containing 2.5ml of drug. It has an easy-to-open safety seal very much like that found on the Xalatan bottle.
Chloramphenicol
This drug is a workhorse in combating bacterial conjunctivitis in many countries throughout the world. Its use in the United States is markedly limited because of the remote possibility of causing aplastic anemia. Chloramphenicol is a highly lipophilic drug with excellent corneal penetration and broad-spectrum coverage. Its mechanism of action is inhibiting bacterial protein synthesis. It is generically available in both solution and ointment forms. Because of (probably unfounded) medicolegal concerns and the availability of many excellent antibiotic options, chloramphenicol is rarely prescribed in the U.S.
The Aminoglycosides
Aminoglycosides are a class represented by gentamicin, tobramycin, and neomycin. The first two are the only members of this class with broad-spectrum antibiotic properties, which allows them to function as standalone drugs. The aminoglycosides are not used systemically (because they can cause ototoxicity) and therefore, they have not had their antibiotic properties compromised by widespread primary care use. They exert their bactericidal action through the inhibition of bacterial protein synthesis. Both gentamicin and tobramycin perform about the same, except that tobramycin appears to be even less likely than gentamicin to cause any epitheliotoxic response. While all aminoglycosides have the potential to cause ocular surface toxicity, this is not a practical concern when used for a short time, as they would be rationally prescribed in eye care (i.e., seven to 10 days), unless the ocular surface was already compromised prior to the institution of treatment. These drugs are generically available in 5ml bottles.
 They are excellent, broad-spectrum
antibiotics. Like the fluoroquinolones,
their forte is in the
gram-negative spectrum, and the
highest MICs are for streptococcal
pathogens. Also like the fluoroquinolones,
these two drugs
should be dosed frequently
(every one to two hours initially
until the infection comes under
control), then the dosing frequency
can be reduced as appropriate
for the amount of time
deemed necessary to achieve a
clinical cure, usually seven to 10
days.
They are excellent, broad-spectrum
antibiotics. Like the fluoroquinolones,
their forte is in the
gram-negative spectrum, and the
highest MICs are for streptococcal
pathogens. Also like the fluoroquinolones,
these two drugs
should be dosed frequently
(every one to two hours initially
until the infection comes under
control), then the dosing frequency
can be reduced as appropriate
for the amount of time
deemed necessary to achieve a
clinical cure, usually seven to 10
days.
The Fluoroquinolones
Like erythromycin, the oral fluoroquinolones have enjoyed enormous popularity among primary care physicians. This has begun to cause significant resistance to this class of drugs. Most such “resistance” arises from in vitro studies and can usually be overcome clinically because of the huge relative volume of drug-per-surface area achievable on the ocular surface. While there are newer generations of fluoroquinolone (just like newer generations of oral cephalosporins), they are only marginally superior to older ones in clinical performance. Like the aminoglycosides, the fluoroquinolones are concentration- dependent in their bactericidal properties. Ciprofloxacin (Ciloxan, Alcon), ofloxacin (Ocuflox, Allergan) and gatifloxacin (Zymar, Allergan) are all available as a 0.3% concentration; levofloxacin (Quixin, Vistakon Pharmaceuticals) and moxifloxacin (Vigamox, Alcon) are available as a 0.5% concentration; the newly FDA-approved besifloxacin (Besivance, Bausch & Lomb) is available as a 0.6% ophthalmic suspension; and levofloxacin (Iquix, Vistakon Pharmaceuticals) is available as a 1.5% concentration. The 1.5% levofloxacin is FDA approved for bacterial keratitis, and other than ofloxacin and ciprofloxacin, is the only fluoroquinolone specifically FDA approved for this purpose.
So what does all this mean clinically? Not very much. When a fluoroquinolone is deemed the class of choice for a particular infectious condition, it is not particularly the specific drug chosen that matters as much as how frequently the drug is dosed. For example, an article in the September 2007 issue of Ophthalmology, compared 1% moxifloxacin, fortified tobramycin/cephazolin, and 0.3% ofloxacin in treating bacterial keratitis.3 The result: they all performed equally. This is one example—of many—of why it is so important for O.D.s to consistently read the literature.
In summary, the topical antibiotics are grossly overutilized—in optometry, ophthalmology, and general medicine. Make every effort to pinpoint an accurate diagnosis (which, in most cases of acute red eye, is not of bacterial etiology), and then select an appropriate drug or drug class to achieve renormalization of tissues.
The frequency of instillation is almost always more important than the drug selected.
As best as we can determine, the four best drugs to combat acute bacterial infection in adults are: bacitracin/polymyxin B/neomycin; tobramycin; 0.6% besifloxacin; and 1.5% levofloxacin.
In children, we use either generic trimethoprim/polymyxin B or topical azithromycin.
The best, general-purpose ophthalmic ointment is a combination of bacitracin with polymyxin B. Only in advanced ocular surface infection would we use eye drops hourly and an ointment at bedtime; otherwise ointments are largely limited to blepharitis care.
We are fortunate to have such an awesome arsenal of medicines available to treat bacterial infections. Use them wisely, judiciously— and aggressively when indicated.
- Abelson MB, Heller W, Shapiro AM, et al; AzaSite Clinical Study Group. Clinical cure of bacterial conjunctivitis with azithromycin 1%: vehicle-controlled, double-masked clinical trial. Am J Ophthalmol. 2008 Jun;145(6):959-65.
- Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008 Jun;145(6):951-958. Epub 2008 Mar 28.
- Constantinou M, Daniell M, Snibson GR, et al. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology. 2007 Sep;114(9):1622-9.

