The past decade has brought eye care practitioners a plethora of new research on dry eye, meibomian gland dysfunction (MGD) and contact lens discomfort. The proliferation of research into these areas is driven by the pervasive nature of ocular surface disease (OSD). The complex and often overlapping nature of the multifactorial conditions that cause dry eye has given rise to a multitude of involved organizational flowcharts, diagnostic tests and treatment paradigms.1-3
This article aims to make sense of the available knowledge concerning MGD and the related diagnostic technologies available.4
A brief review of the research, clinically available devices and emerging technologies is presented to deepen our understanding.
Understanding the Elements
Dry eye disease (DED) is one type of OSD and has been broadly classified into two categories: aqueous-deficient and evaporative.5 MGD largely contributes to the latter, although it is possible to see patients with mixed presentations.6
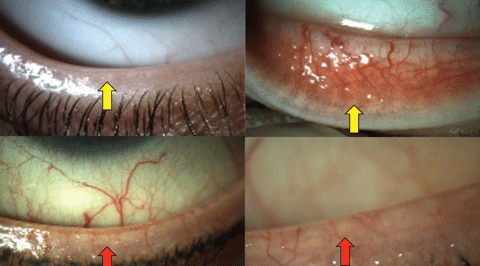 |
| Fig. 1. Normal lower lid appearance, indicating well-defined meibomian gland orifices (top left) and ductal elements (top right) highlighted with yellow arrows. Contrast the normal appearance of the lids above with the telangiectasia, posterior dragging and loss of anatomical detail in the lower images (red arrows) in obstructive MGD. Click image to enlarge. |
Researchers suggest that MGD may be the leading cause of DED throughout the world.7 Due to considerable variation in diagnostic criteria and methodological differences in various studies, its exact prevalence has not yet been clearly established.8
Classification
A simple classification system has been devised for MGD based on whether or not the glands under- or over-produce meibum of varying quality. These states have been termed “low delivery”—if the patient has an undersecretion of meibum, either with or without obstruction—or “high delivery”—an oversecretion of meibum.7,9 The end result of either gives rise to a cascade of tear film alteration, irritation, inflammation and OSD we see manifested clinically as dry eye.7
Table 1. Obstructive Disease Types | |
| Condition | Gauging the likelihood of a clinical encounter |
| Trachoma | A common cause of cicatricial changes in underdeveloped countries but rare in the United States, with some isolated occurrences in native American populations. One study suggests 22.4% of patients with scarring will have dry eye complaints.55 |
| Ocular cicatricial pemphigoid (OCTT) | OCTT occurs at a rate of 1/12,000 to 1/60,000.17,56 |
| Erythema multiforme (EM) | Incidence per year is less than 1%; 70% of recurrent EM cases have herpes simplex.17,57,58 |
| Acne rosacea | Up to 50% of rosacea patients may have MGD.59,60 |
| Atopic dermatitis | Prevalence in adults 10.2%.39 |
| Psoriasis | Psoriatic are at risk for obstructive MGD.40 |
| Seborrhea | Up to 46% of patients with MGD may have seborrhea.18,60 |
Anatomy and Pathophysiology
Approximately 30 meibomian glands can be found in the upper lid and 25 in the lower lid, with a correspondingly higher volume of acinar tissue in the upper lid.10-12 These sebaceous glands produce the lipids and proteins that are secreted at the lid margin just posterior to the cilia and anterior to the mucocutaneous junction under the control of complex neuromuscular and hormonal interactions (Figure 1).13
Low delivery states may arise from reduced secretion with or without gland obstruction—the former being more common.9,14,15 Obstruction of the ducts occurs as tissues undergo keratinization and may or may not involve cicatricial changes which can be differentiated clinically by noting whether the orifices remain in their normal anatomical positions or are displaced posteriorly toward the mucocutaneous junction (Figure 1).4,16 The causes for non-obstructive states have not been well established.9 Etiological factors observed in cicatricial obstructive disease include infectious (trachoma), autoimmune (ocular pemphigoid), immune (erythema multiforme) and hypersensitivity (atopy) conditions.17 Non-cicatricial obstructive disease is more commonly associated with inflammatory conditions such as seborrheic or psoriatic dermatoses, atopy and acne rosacea (Table 1).17
High delivery states represent an excessive release of meibum and have also been associated with acne rosacea, atopy and seborrheic dermatitis, but without signs of obstruction.18
Evaluating the Problem
Understanding the organizational schema, cascade of subsequent events and anatomy and pathophysiology, which gives rise to MGD, is the starting point for the work-up. This framework allows clinicians—and industry—to develop tools for its systematic diagnosis and management.
Diagnostic approach—A comprehensive evaluation of the meibomian glands includes a balanced assessment of both the structure and function (Table 2).
Your approach should exclude causes of aqueous deficient dry eye (ADDE) prior to arriving at a diagnosis of evaporative dry eye secondary to MGD. You should first perform testing techniques that are noninvasive to avoid distorting your observations. Invasive techiques that manipulate the lids may express meibum and alter the test results. Many of the tests are indirect measures of function. Signs may correlate poorly with symptoms, and correlations between different tests—and repeatability for the same test—may vary.20-26
Biomicroscopy and expression—Begin your assessment of the meibomian gland with a careful slit lamp evaluation, looking for telangiectasia, hyperemia, capping and anatomical distortions (Figure 1). Assess any tear film debris before attempting to express the glands or performing invasive tests. Gland expression can be done digitally, with cotton tip applicators or with one of a number of devices. The goal is to milk the meibum out of the glands from their point of origin towards the orifices. The control that the clinican is able to exert and the efficacy and discomfort a patient experiences will vary somewhat with each device. The meibum should be clear and flow easily rather than be turbid or paste-like.
Varying scales have been proposed to assess the number of glands expressed and the quality of the secretions in a research environment.27,28,29 One scale with potential clinical use suggests an approach based on the number of glands which can be expressed on the lower lid in a descending manner:
• 4 (or more)=normal,
• 3=mildly reduced,
• 2=moderately reduced,
• 1 (or less)=severely reduced.27
Interferometry—This test provides insights into lipid layer thickness, stability of the tear film, proving useful in assessing the contribution of the meibomian glands to DED.30-31 The only commercially available device within the United States is the LipiView II unit (TearScience). Absolute cut-off values have not been clearly established and may vary over time or be influenced by inadvertent expression of the meibomian glands or blinking.32,33 However, they are likely in the range from a low of 54nm in Asians to 75nm in a cross section of a clinic population.33,34
Table 2. Assessing Meibomian Gland Function and Structure | ||
| Technique | Purpose | Interpretation |
| Schirmer and tear film break up time20,25,62,63 | Exclude causes of aqueous deficient dry eye disease (tear production/evaporation). | Decreased wetting (<5mm in five minutes) and tear film instability (<10 seconds). |
| Biomicroscopy with expression27,64 | Identify signs of cicatricial and inflammatory changes, which may lead to either obstructive or non-obstructive dysfunction. | Notching, telangiectasia, hyperemia, capping, turbid or paste-like secretions or an absence of secretions. |
| Stains and dyes (fluorescein, lissamine green, rose begal)5,20,65,66 | May be used to assess tear film break up times; apoptosis, dead or devitalized cells and areas where the tight junctions between epithelial cells may be diminished. | Rapid break-up times; punctate, patchy or regional staining of the cornea especially inferiorly or bulbar conjunctiva; staining of the lid-wiper region. |
| Lipid layer interferometry39,31,34 | Helps determine if the lipid layer in the tear film is sufficiently thick to avoid evaporation and instability. | A reduced thickness has been correlated with MGD. |
| Osmolarity1,67,68 | Nonspecific clinical measure of tear film osmolarity. Research devices are more accurate. | Any decrease in meibum or aqueous secretion contributes to increases in osmolarity. |
| Meibometry69,70 | A research technique used to assess basal meibum levels. | Photometric assessments of interaction between the meibum and a reactive substrate on a tape provide insight, but the test is influenced by many factors. |
| Transillumination36,71 | Allows visualization of gland and ductal morphology by applying a transilluminator to the epidermal side of an everted lid. | Atrophy, “drop-out,” shortening in ductal length, dilation of glands or ducts, and tortuosity may indicate MGD. |
| Infrared (IR) imaging35-72 | Enhances the contrast of the image obtained from transillumination or noncontact tests. | Same as transillumination. |
| Laser confocal microscopy49,73 | Provides a higher resolution image at a microscopic level than available from IR tests. | Similar to transillumination and IR imaging but also allows detection of inflammatory cells and fibrosis. |
| Optical coherence tomography43,54,74 | Allows 2D and 3D assessments of MG volume. | The implication is that a decreased MG volume is a sign of MGD. |
Transillumination and meibography—Transillumination (meiboscopy) and meibography may be performed in a variety of ways (Table 2), using contact or non-contact devices. Regardless of the method, the interpretations are similar. Evaluate the number and morphology of the glands from their point of origin through to the ductal termination at the orifices. As previously noted, anticipate 30 in the upper and 25 in the lower lid.11-13 Ducts should not be attenuated and atrophied (as indicated by “drop-out”) or dilated, which would suggest obstruction with keratinized cells and meibum. 4,16,35-37
Transillumination (meiboscopy) may be performed easily in office without the need of more expensive or sophisticated devices.38 After darkening the exam room, evert the lids (upper and lower in sequence), position your transilluminator against the cutaneous side of the lids and make observations from the palpebral conjunctival side using the white light source.38,39 Note areas where the normal ductal anatomy is absent, tortuous or attenuated as well as areas of drop-out. The shortcomings of this method include a limited field of view, low contrast between structures limiting visualization of details and some discomfort for the patient (Figure 2).
Meibography differs from meiboscopy by using photo or video documentation, or both, of examiner views applying either white or infrared light to increase the contrast of the anatomical detail.39,40 The Lipiview II unit makes use of both reflected infrared light (dynamic reflected illumination) and transilluminated (adaptive high-definition transillumination) images.41 There is contact made between the transilluminator and the lids. Another device is the Keratograph 5M (Oculus, Wetzlar, GE).42 Its Meibo-Scan software analyzes the reflected image from an 840nm diode source to provide a high contrast image of the everted lids.42 This is a non-contact device (Figure 3). The Lipiview II unit is part of a dedicated dry eye diagnosis and treatment platform whereas the Keratograph 5M has multiple configurations, which include dry eye reports, tear film scans, meibomian gland scans, topography, pupilometry, imaging and oxygen transmissibility mapping through a soft contact lens.
Both of these devices are convenient, easy to use and comfortable for the patient.
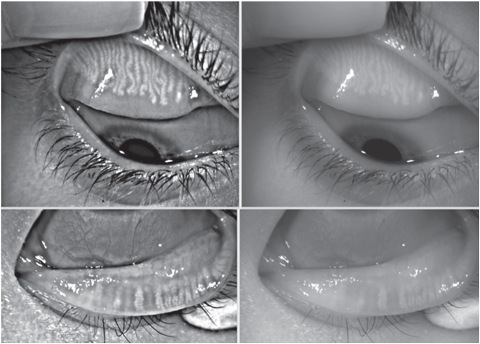 |
| Fig. 3. The reflected IR images (left) and white light images (right) in a patient with MG attenuation and drop-out. Click image to enlarge. |
Multiple grading systems are currently competing for prominence in analyzing meibography images.43,44 Scales vary widely based on methodology and are primarily used in a research setting. In general, they attempt to assess the amount of drop-out in relation to the area of the lid assessed.43-49 Some of these scales are fairly complex regarding the angle of deviation of the glandular ducts and whether or not changes in the acini are noted.44 With the absence of a standard of care, clinicians are more focused on detection and change over time in a qualitative sense. Inter- and intra-reliabilities are “moderate to fair” by some estimates—combining upper with lower lid observations may be the most useful approach.46,47 Research shows computerized grading systems have the best repeatability in contrast to five- or four-grade scales.48 As of yet, no commercial device offers an option similar to normative databases used in threshold visual fields and optical coherence tomography of the posterior segment.
The Future, Confocal Microscopy and 3D-OCT
Some of the devices that we may eventually find commonplace are now prominent only in the realm of research and large academic centers. Clinicians frequently see adapted versions of research devices once proven merit gives way to larger scale production that brings down the acquisition cost.
 |
| Fig. 2. Transillumination (meiboscopy) of the lower lids shows a normal arrangement of meibomian glands. Click image to enlarge. |
Confocal microscopes, for example, are typically found in large research clinics or teaching programs. With these devices, the lids are everted and a cap with a gel interface is applanated against the lid surface, providing the observer with a view of the acinar structures of the MGs.49-53 At least one unit is available with software that can quantify morphological changes with a high degree of sensitivity and specificity.19,52,53
3D ultrahigh-resolution OCT volumetric imaging of the meibomian glands in healthy and inflamed states was first reported in 2010 and demonstrated a future potential for yet another tool to evaluate morphology.54
The “take-away” for eye care clinicians is that meibomian gland dysfunction plays a predominant role in evaporative dry eye and contributes to ocular surface disease. A basic understanding of how to assess the morphological changes underlying the clinical signs and symptoms and establishing clinical benchmarks are excellent ways of tracking the changes over time and assessing the effectiveness of therapies. Staying informed about the scientific evidence, diagnostic and management strategies as they emerge allows us to better serve our patients while expanding growth opportunities for our practices.
Dr. Fuller is an associate professor and founding supervisor of the Cornea & Contact Lens – Refractive Surgery residency at The Eye Center, Southern College of Optometry.
|
1. DEWS. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul Surf. 2007 Apr;5(2):75-92. 2. Asbell P, Stapleton F, Wickström K, et al. The international workshop on meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011 Mar 30;52:2065-85. 3. Nichols J, Jones L, Nelson D. The TFOS international workshop on contact lens discomfort. Invest Ophthalmol Vis Sci. 2013;54:TFOS1–122. 4. Korb D, Henriquez A. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51:243–51. 5. Lemp M. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J 1995;21:221–32. 6. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul Surf. 2007;5:75–92. 7. Nichols K, Foulks G, Bron A, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–9. 8. Schaumberg D, Nichols J, Papas E, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005. 9. Nelson J, Shimazaki J, Benitez-del-Castillo J, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–7. 10. Bron A, Tiffany J, Gouveia S, et al. Functional aspects of the tear film lipid layer. Exp Eye Res 2004;78:347–60. 11. Knop N, Knop E, Millar T. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011 Mar; 52(4): 1938–78. 12. Greiner J, Glonek T, Korb D, et al. Volume of the human and rabbit meibomian gland system. Adv Exp Med Biol 1998;438:339–43. 13. Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–78. 14. Obata H, Horiuchi H, Miyata K, et al. Histopathological study of the meibomian glands in 72 autopsy cases. Nihon Ganka Gakkai Zasshi. 1994;98:765–71. 15. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol (Chicago, Ill 1960) 1995;113:1266–70. 16. Henriquez A, Korb D. Meibomian glands and contact lens wear. Br J Ophthalmol 1981;65:108–11. 17. Bron A and Tiffany J. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–65. 18. McCulley J, Dougherty J, Deneau D. Classification of chronic blepharitis. Ophthalmology 1982;89:1173–80. 19. Wise R, Sobel R, Allen R. Meibography: A review of techniques and technologies. Saudi J Ophthalmol Off J Saudi Ophthalmol Soc. 2012;26:349–56. 20. Zeev M, Miller D, Latkany R. Diagnosis of dry eye disease and emerging technologies. Clin Ophthalmol. 2014;8:581–90. 21. Alves M, Reinach P, Paula J. Comparison of diagnostic tests in distinct well-defined conditions related to dry eye disease. PLoS One 2014;9:e97921. 22. Onwubiko S, Eze B, Udeh N, et al. Dry eye disease: Concordance between the diagnostic tests in African eyes. Eye Contact Lens. December 2015. 23. Schiffman R, Christianson M, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–21. 24. Cox S, Nichols K, Nichols J. Agreement between automated and traditional measures of tear film breakup. Optom Vis Sci. 2015;92:e257–63. 25. Nichols K, Mitchell G, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23:272–85. 26. Powell D, Nichols J, Nichols K. Inter-examiner reliability in meibomian gland dysfunction assessment. Invest Ophthalmol Vis Sci. 2012;53:3120–5. 27. Meadows J, Ramamoorthy P, Nichols J. Development of the 4-3-2-1 meibum expressibility scale. Eye Contact Lens. 2012 Mar;38(2):86-92. 28. Arita R, Itoh K, Maeda S. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009;116:2058–63.e1. 29. Bron A, Benjamin L, Snibson G. Meibomian gland disease. Classification and grading of lid changes. Eye(Lond). 1991;5 (Pt. 4):395–411. 30. Eom Y, Lee J, Kang S, et al. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am J Ophthalmol. 2013;155:1104–10.e2. 31. Blackie C, Solomon J, Scaffidi R, et al. The relationship between dry eye symptoms and lipid layer thickness. Cornea 2009;28:789–94. 32. Finis D, Pischel N, Borrelli M. Factors influencing the measurement of tear film lipid layer thickness with interferometry. Klin Monbl Augenheilkd. 2014;231:603–10. 33. Zhao Y, Tan C, Tong L. Intra-observer and inter-observer repeatability of ocular surface interferometer in measuring lipid layer thickness. BMC Ophthalmol 2015;15:53. 34. Finis D, Pischel N, Schrader S. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for meibomian gland dysfunction. Cornea 2013;32:1549–53. 35. Robin J, Jester J, Nobe J. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. A clinical study. Ophthalmology. 1985;92:1423–6. 36. Jester J, Rife L, Nii D. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982;22:660–7. 37. Koh Y, Celik T, Lee H. Detection of meibomian glands and classification of meibography images. J Biomed Opt. 2012;17:086008. 38. Tapie R. Etude biomicroscopique des glandes de meibomius. Ann Ocul 1977;210:637–48. 39. Wise R, Sobel R, Allen R. Meibography: A review of techniques and technologies. Saudi J Ophthalmol Off J Saudi Ophthalmol Soc. 2012;26:349–56. 40. Ngo W, Srinivasan S, Jones L. Historical overview of imaging the meibomian glands. J Optom 2013;6:1–8. 41. Tear Science Brochure. TearScience Solut MGD 2016:1–6. 42. The Oculus Keratograph 5M. Oculus Prod 2016. 43. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–5. 44. Srinivasan S, Menzies K, Sorbara L. Infrared imaging of meibomian gland structure using a novel keratograph. Optom Vis Sci 2012;89:788–94. 45. Bailey I, Bullimore M, Raasch T, Taylor H. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci 1991;32:422–32. 46. Nichols J, Berntsen D, Mitchell G, Nichols K. An assessment of grading scales for meibography images. Cornea 2005;24:382–8. 47. Pult H, Riede-Pult B, Nichols J. Relation between upper and lower lids’ meibomian gland morphology, tear film, and dry eye. Optom Vis Sci. 2012;89:e310–5. 48. Pult H, Riede-Pult B. Comparison of subjective grading and objective assessment in meibography. Cont Lens Anterior Eye. 2013;36:22–7. 49. Matsumoto Y, Sato E, Ibrahim O, et al. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–71. 50. Matsumoto Y, Shigeno Y, Sato E, et al. The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefe’s Arch Clin Exp Ophthalmol. Albr von Graefes Arch für Klin und Exp Ophthalmol. 2009;247:821–9. 51. Wakamatsu T, Sato E, Matsumoto Y, et al. Conjunctival in vivo confocal scanning laser microscopy in patients with Sjögren syndrome. Invest Ophthalmol Vis Sci 2010;51:144–50. 52. Ngo W, Srinivasan S, Jones L. Historical overview of imaging the meibomian glands. J Optom 2013;6:1–8. 53. Ibrahim O, Matsumoto Y, Dogru M, et al. The efficacy, sensitivity and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117:665–72. 54. Bizheva K, Lee P, Sorbara L, Hutchings N, Simpson T. In vivo volumetric imaging of the human upper eyelid with ultrahigh-resolution optical coherence tomography. J Biomed Opt. 15:040508. 55. Lucena A, Akaishi P, Rodrigues M. Upper eyelid entropion and dry eye in cicatricial trachoma without trichiasis. Arq Bras Oftalmol. 2012;75:420–2. 56. Kirzhner M, Jakobiec F. Ocular Cicatricial Pemphigoid: A Review of Clinical Features, Immunopathology, Differential Diagnosis, and Current Management. Semin Ophthalmol September 2011. 57. Staikuniene J, Staneviciute J. Long-term valacyclovir treatment and immune modulation for Herpes-associated erythema multiforme. Cent J Immunol/Polish Soc Immunol Elev other Cent Immunol Soc 2015;40:387–90. 58. Sokumbi O, Wetter D. Clinical features, diagnosis and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol 2012;51:889–902. 59. Machalinska A, Zakrzewska A, Markowska A, et al. Morphological and functional evaluation of meibomian gland dysfunction in rosacea patients. Curr Eye Res December 2015:1–6. 60. McCulley J, Dougherty J. Blepharitis associated with acne rosacea and seborrheic dermatitis. Int Ophthalmol Clin 1985;25:159–72. 61. Silverberg J, Hanifin J. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132–8. 62. Schirmer O. Studien zur physiologie und pathologie der trenasbsonderung and treabfuhr. Arch Klin Exp Ophthalmol.1903;56:197–291. 63. Lemp M. Breakup of the tear film. Int Ophthalmol Clin. 1973;13:97–102. 64. Korb D and Blackie C. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea 2008;27:1142–7. 65. Yoon K, Im S, Kim H, You I. Usefulness of double vital staining with 1% fluorescein and 1% lissamine green in patients with dry eye syndrome. Cornea. 2011;30:972–6. 66. Bron A, Evans V, Smith J. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50. 67. Gilbard J, Rossi S, Heyda K. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology 1989;96:1180–6. 68. Versura P, Profazio V, Campos E. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res 2010;35:553–64. 69. Yokoi N, Mossa F, Tiffany J, Bron A. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol. (Chicago, Ill 1960) 1999;117:723–9. 70. Yokoi N, Komuro A. Non-invasive methods of assessing the tear film. Exp Eye Res 2004;78:399–407. 71. Kohno T, Ohnishi Y. [In vivo transillumination of the meibomian gland in yusho patients]. Fukuoka Igaku Zasshi. 1987;78:355–7. 72. Satjawatcharaphong P, Ge S, Lin M. Clinical outcomes associated with thermal pulsation system treatment. Optom Vis Sci. 2015;92:e334–41. 73. Qazi Y, Aggarwal S, Hamrah P. Image-guided evaluation and monitoring of treatment response in patients with dry eye disease. Graefes Arch Clin Exp Ophthalmol. 2014;252:857–72. 74. Palamar M, Degirmenci C, Ertam I, Yagci A. Evaluation of dry eye and meibomian gland dysfunction with meibography in patients with rosacea. Cornea. 2015;34:497–9. |

