Dry eye syndrome (DES) is a chronic disease, one that has been estimated to affect more than 3.2 million middle-aged and older women and nearly 1.7 million men in the United States.1,2, Because the disorder is seen more frequently in older people, these figures can be expected to increase exponentially as our population ages.3 Between half and three-quarters of all contact lens wearers suffer from dry eye symptoms, and about a third of people who have undergone refractive surgery still have DES six months after the procedure.4 A chronic disease, DES accounts for about one-fifth of all visits to eye care professionals.5-8
Clearly, effective treatments are needed for DES, yet a recent survey found that, due to dissatisfaction with existing treatments, 94% of eye care practitioners wanted more treatment options.9 To that end, a cellulose ophthalmic insert has been re-introduced in the hope that this recent addition to the armamentarium of DES options will help better meet the needs of some patients diagnosed with moderate-to-severe DES, especially those who have tried artificial tears for an adequate period of time and remain symptomatic.
The product, called Lacrisert (Aton Pharma), is an insert made of 5mg of hydroxypropyl cellulose that is 1.27mm in diameter and 3.5mm long. With the aid of a reusable applicator, each insert is placed into the inferior cul-de-sac of the eye beneath the base of the tarsus. There, it begins to soften within minutes, dissolving over the course of about 12 hours, thickening the precorneal tear film and protecting the eye.10
Methodology
Of course, the use of hydroxypropyl cellulose ophthalmic inserts is not a new concept. They were first approved by the FDA more than 25 years ago, and have already undergone scrutiny in controlled studies.11,12
More recently, though, a multicenter, open-label registry that enrolled 520 patients evaluated their effectiveness, ease of use and acceptability under real-life conditions.13-14
Participating investigators, consisting of ophthalmologists and optometrists, could maintain their chosen standard of care, adding the insert to existing therapy to determine if there was incremental benefit, or changing treatment and using the inserts as monotherapy. This type of study gives us a look at clinical practices in action.
Patients in this registry had bilateral moderate-to-severe DES and a history of artificial tear use or a desire to use them within the week prior to participating in the study. Those taking systemic or topical medications to treat clinically significant blepharitis, meibomian gland dysfunction, or lid margin inflammation and those who had ongoing bacterial, viral or fungal ocular infection were excluded. Other exclusions were patients who had active ocular inflammation, preauricular lymphadenopathy, LASIK surgery within 12 months of their first visit, ocular surgical intervention within three months of the beginning of the study, or a systemic disease that the investigator felt could interfere with study measurements or patient compliance. The registry did not exclude patients who were currently using cyclosporine ophthalmic emulsion or those fitted with punctal plugs at the time of enrollment.
The registry consisted of two office visits, four weeks apart. At the first visit, investigators collected patient information and examined patients for DES, using the methods generally employed in their practices. Participants completed a questionnaire that evaluated their symptoms, activities of daily living and quality of life. This questionnaire incorporated the
validated Ocular Surface Disease Index (OSDI), which measures DES severity, vision-related function, symptoms and environmental triggers, and yields a normal (0-12), mild (13-22), moderate (23-32), or severe (33-100) disease score.15

Also at this visit, patients were shown how to place the inserts properly, viewed an instructional video, positioned the inserts as the investigator or coordinator watched, were told to use the inserts once daily during the study, and were scheduled for the second visit.
Three days after the first visit, patients received a follow-up telephone call to assess adverse events, determine if the inserts were being used properly, and reinstruct if necessary.
At the second visit (one month later), investigators reviewed adverse events and reevaluated the patients to determine changes in DES signs and symptoms. Investigators also completed a Physician Questionnaire. Patients completed a similar questionnaire to the one at the first visit, as well as an assessment of patient acceptability and satisfaction.
The Results
Mean OSDI total scores improved by 21%, falling from the severe range (41.8 ±22.38) at the first visit into the moderate range (32.9 ± 21.97) at the second. Although the OSDI provides a total score, there were significant reductions in the frequency of sensitivity to light (24%), grittiness (34%), and painful or sore eyes (42%). There were also nonsignificant increases of 6.4% for blurred vision and 12.7% for poor vision.
The frequency of difficulties in all four areas of activities of daily living measured by the OSDI improved significantly as well: reading by 13%, driving at night by 25.9%, working with a computer or ATM by 21.9%, and watching television by 19%.
The OSDI also measured discomfort under various environmental conditions. In windy conditions, discomfort decreased by 33%, in conditions of low humidity by 38%, and in air conditioning by 31%.
Not only were there significant reductions in the severity of symptoms of discomfort, burning, dryness, grittiness, sensitivity to light, and stinging, but activities of daily living improved as well. Patients reported significantly increased ability to read (15%), watch TV (28%) or movies (31%), and do housework (38%). Patients were also better able to perform tasks in heated areas.
The desire to use additional treatment to reduce symptoms decreased from 50% of patients at the first visit to 20% at the second visit.
Patient Assessment
Almost all of the registry participants were unfamiliar with the inserts before this study, and when asked to rate their satisfaction at visit two, half of all enrollees rated themselves satisfied to most satisfied, and 53% felt that the inserts improved the effectiveness of their existing therapy. Five people (0.96%) reported foreign body sensation that led to discontinuation, and five people (0.96%) experienced ocular discomfort.
Clinician Assessment
The responses of investigators to this product were very positive as well. They noted significant improvements in measurements of tear film breakup time (TFBUT), fluorescein staining and Schirmer’s test tear volume.
When asked to compare the inserts with artificial tears, 53% felt the inserts were more effective, and 91% thought their effects were longer lasting. Almost 84% said that they would recommend the inserts for patients with moderate-to-severe DES, and nearly 88% said they would consider using the inserts as part of an overall DES treatment regimen.
Safety Profile
Patients diagnosed with keratitis sicca are good candidates for therapy that includes ophthalmic inserts.
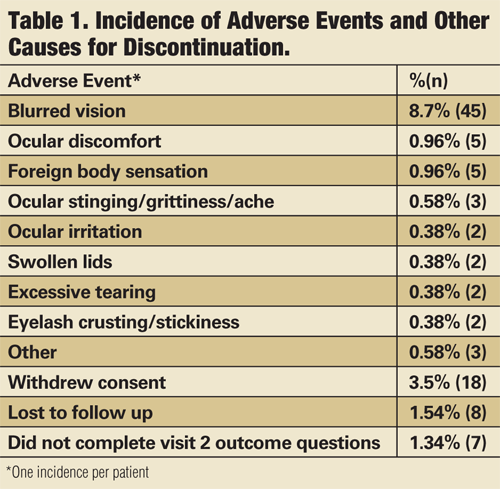
Of the 520 patients enrolled in the registry, 69 (13%) discontinued the inserts due to an adverse event. The most common adverse event was blurred vision, which led to discontinuation in 45 (9%) patients. Other adverse events that led to discontinuation occurred less than 1% of the time, and there was one corneal abrasion, unrelated to insert use (see, “Incidence of Adverse Events and Other Causes for Discontinuation”).
Concerns and Limitations
With a slow-release formulation, blurring can be expected, and usually occurs right after the insert is placed. This is due to an increase in tear volume, which provides added ocular surface protection.10 For the most part, blurring is transient, and the addition of artificial tears will thin the tear film sufficiently. As an alternative, the insert can be placed before going to bed.
The second concern involved difficulty in using the product. This problem can be overcome simply by having a technician or assistant instruct the patients in placing the inserts, observe as the patients do it by themselves, and then follow up in a couple of days. It is not hard to learn how to use the inserts; in fact, in a study of people with hand deformities secondary to rheumatoid arthritis, only 9% were unable to use the applicator to position the insert correctly.16
One limitation of the study was that investigators were not asked to report the established therapeutic regimens that were being continued. Thus, we don’t know exactly how much incremental benefit the inserts provided beyond the established regimen. What we do know is that at the first visit, about half of all patients felt a frequent need to use dry eye treatments for symptom relief. By visit two, with the use of the inserts, about 20% of the patients felt the need to use additional dry eye treatments.14
Chronic conditions, such as DES, are difficult to treat, but cellulose ophthalmic inserts—alone or with existing treatment—are a valuable option for managing moderate-to-severe DES.
Dr. Koffler is the founder and Medical Director of Koffler Vision Group, in Lexington, Ky. He is the lead investigator of the Lacrisert study group and is on the speaker’s bureau and advisory board for Aton Pharma. Dr. Karpecki is the Cornea Services and Clinical Research Director at the Koffler Vision Group, and is the co-editor of Review’s “Research Review” column. Dr. Karpecki has no financial interests in this product.
1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318-326.
2. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127(6):763-768.
3. National Eye Institute. Dry Eye. National Institutes of Health: Bethesda, MD; 2009. Available at www.nei.nih.gov. (Accessed February 2, 2010.)
4. The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93-107.
5. Optometric Clinical Practice Guideline. Care of the patient with ocular surface disorders. St Louis, MO: American Optometric Association; 2002.
6. Smith J, Nichols KK, Baldwin EK. Current patterns in the use of diagnostic tests in dry eye evaluation. Cornea. 2008;27:656-662.
7. Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):108-152.
8. Khanal S, Tomlinson A, McFadyen A, et al. Dry eye diagnosis. Invest Ophthalmol Vis Sci. 2008;49:1407-1414.
9. Asbell PA, Spiegel S. Opthalmologists perceptions regarding treatment of moderate to severe dry eye: results of a physician survey. Eye Contact Lens. 2010;36(1):33-38.
10. Lacrisert [package insert]. Lawrenceville, NJ: Aton Pharma, Inc; 2007.
11. Katz JI, Kaufman HE, Breslin C, Katz IM. Slow-release artificial tears and the treatment of keratitis sicca. Ophthalmology. 1978;85(8):787-793.
12. Werblin TP, Rheinstrom SD, Kaufman HE. The use of slow-release artificial tears in the long-term management of keratitis sicca. Ophthalmology. 1981;88(1):78-81.
13. McDonald M, D’Aversa G, Perry HD, et al. Hydroxypropyl cellulose ophthalmic inserts (lacrisert) reduce the signs and symptoms of dry eye syndrome and improve patient quality of life. Trans Am Ophthalmol Soc. 2009;107:214-221.
14. Koffler BH, McDonald M, Nelinson DS; LAC-07-01 Study Group. Improved signs, symptoms, and quality of life associated with dry eye syndrome: hydroxypropyl cellulose ophthalmic insert patient registry. Eye Contact Lens. 2010 May;36(3):170-6.
15. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615-621.
16. Hill JC. Slow-release artificial tear inserts in the treatment of dry eye in patients with rheumatoid arthritis. Br J Ophthalmol. 1989;73:151-154.
17. Johnson ME, Murphy PJ. The agreement and repeatability of tear meniscus height measurement methods. Optom Vis Sci. 2005;82(12):1030-1037.
18. Perry HD. Dry eye disease: pathophysiology, classification, and diagnosis. Am J Manag Care. 2008;14:S79-S87.
19. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762-770.
20. Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146:350-356.
21. Buchholz P, Steeds CS, Stern LS, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf. 2006;4(3):155-161.
22. Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412-1419.

