 A 24-year-old pregnant Indian woman presents for examination, complaining of reduced vision in her right eye since awakening that morning. She claims that she sees a big hazy bubble when she covers her left eye. She denies any pain, photophobia or photopsiae. Her ocular history is unremarkable; she has never worn any corrective glasses and has never had any injuries or medical problems concerning her eyes.
A 24-year-old pregnant Indian woman presents for examination, complaining of reduced vision in her right eye since awakening that morning. She claims that she sees a big hazy bubble when she covers her left eye. She denies any pain, photophobia or photopsiae. Her ocular history is unremarkable; she has never worn any corrective glasses and has never had any injuries or medical problems concerning her eyes.
She reports no other symptoms and claims to have had an uncomplicated pregnancy through the first two trimesters. She uses no medications.
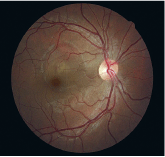
Upon examination, best-corrected visual acuity is 20/30 O.D. (with slight hyperopic correction) and 20/20 O.S. Her pupils, ocular motility, confrontation fields, biomicroscopy and tonometry are all normal. But, fundus examination reveals a well-circumscribed retinal elevation approximately 5 disc diameters in size encircling the right fovea, characteristic of central serous chorioretinopathy (CSC). Optical coherence tomography (OCT) confirms a bullous separation between the sensory retina and underlying retinal pigment epithelium.
Not Just Type A
CSC presents as a focal, serous detachment of the neurosensory retina within the posterior pole. While foveal involvement is the rule, the exact center of the lesion and the maximum height of the detachment may be extrafoveal.1 By definition, CSC involves a defect at the level of the retinal pigment epithelium (RPE); this can sometimes be viewed ophthalmoscopically as one or more yellow areas, or even a small concurrent RPE detachment.1 OCT allows practitioners to better study CSC, and research using this technology suggests that RPE detachments may be present in most cases, even though they may not be readily identifiable upon direct observation.2
This 24-year-old pregnant woman presented with an unusual finding: central serous chorioretinopathy.
When we think of CSC, we typically envision a Type A (e.g., impatient, highly competitive, business-oriented individual) male patient between the ages of 30 and 50 under substantial psychological stress.1,3,4 While this is the stereotypical presentation, take note that both genders may be affected and that a host of other factors, both endogenous and exogenous, may contribute. Women account for 12% to 28% of the affected population.1,4-7 Moreover, pregnancy is a recognized risk factor for CSC, with an identified odds ratio of 7.1 in a case-control study of 312 patients.8 So, be sure to consider this retinal condition in pregnant women who present with sudden-onset visual complaints.
Another risk factor that has been highly publicized in recent years: the use of systemic corticosteroids. Numerous reports of CSC in association with steroid use, including oral, injectible, inhaled and intranasal formulations, have been published.9-11 One of the theories offered for this unique association is glucocorticoid-induced inhibition of collagen production, a main component of Bruchs membrane.12 Another theory is that increased plasma levels of cortisol may propagate permeability alterations in CSC via inhibition of leukocyte migration, fibroblastic proliferation and wound healing.13 Either of these mechanisms may lead to the formation of RPE defects in predisposed individuals, allowing for transudation of serous fluid and detachment of the neurosensory retina. Whatever the pathophysiology, systemic corticosteroids are considered the greatest risk for developing CSC, with an odds ratio of more than 37.8
Additional associated factors have been identified as potentially contributory to CSC development, including antibiotic use, alcohol intake, untreated hypertension and allergic respiratory disease.8 Isolated reports have shown at least an anecdotal relationship between CSC and Viagra (sildenafil, Pfizer), obstructive sleep apnea syndrome and Helicobacter pylori infection.14-16
Testing for CSC
Many sources indicate that fluorescein angiography (FA) offers the only definitive confirmatory test, implying that this technique is the standard of care and must be performed all cases.1,8,17-19
Our experience, however, is that diagnosis of CSC is typically straightforward. In fact, most clinicians can accurately identify this condition based upon the subjective visual complaints and classic ophthalmoscopic appearance. OCT can provide confirmation more safely than FA. For this reason, we tend to refrain from ordering FA routinely, and remain especially vigilant in those cases where FA may pose an unnecessary health risk to the patient (e.g., in pregnant women or cardiac cases). In atypical cases, though, we recommend fluorescein or indocyanine green studies, particularly in those cases involving turbid or sanguineous fluid within the CSC cavity, as this sign may be indicative of subretinal neovascularization.20
Observe or Treat?
Most clinicians take a wait-and-see approach in cases of CSC, because the natural course of this disease typically shows spontaneous resolution within 90 days.21 Patient reassurance, counseling and discontinuation of corticosteroid therapy (when appropriate) may be all that is required and usually lead to complete recovery of both the detachment and visual acuity.
But, cases that persist beyond three or four months may warrant intervention, and this is the point at which therapeutic options should be considered and discussed.
Historically, laser photocoagulation has been the most widely utilized treatment for persistent CSC, when there is an identifiable RPE abnormality causing the condition. Prospective studies have shown that photocoagulation can shorten the duration of the condition and reduce the likelihood of recurrences, although complications may occur; laser-induced damage to Bruchs membrane may (in rare cases) lead to the induction or exacerbation of subretinal neovascularization.1,22 Also, subthreshold diode micropulse laser photocoagulation may reduce some of the risks associated with more traditional argon laser therapy.23
Recently, newer treatments have been tried for cases of chronic, unrelenting CSC, including photodynamic therapy with verteporfin, transpupillary thermotherapy and intravitreal bevacizumab.24-26 Attempts have also been made to use systemic antiglucocorticoid therapies, such as ketoconazole and mifepristone, to alleviate CSC.27,28 While no treatment option is 100% effective, these newer alternatives may hold some promise for that small percentage of patients who fail to respond to more conventional therapy.
Overall, CSC is usually not a serious concern for practitioners, despite its dramatic presentation. The key to managing this disorder is to identify it early, provide thorough patient counseling and careful follow-up, and when necessary, comanage the patient with an experienced retinologist.
1. Wang M, Sander B, la Cour M, Larsen M. Clinical characteristics of subretinal deposits in central serous chorioretinopathy. Acta Ophthalmol Scand 2005 Dec;83(6):691-6.
2. Mitarai K, Gomi F, Tano Y. Three-dimensional optical coherence tomographic findings in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2006 Nov; 244(11):1415-20.
3. Wynn PA. Idiopathic central serous chorioretinopathya physical complication of stress? Occup Med (Lond) 2001 Mar;51(2):139-40.
4. Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol 1999 Jul;128(1):63-8.
5. Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol 1992;81(4):379-86.
6. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996 Dec;103(12):2070-9; discussion 2079-80.
7. Spitznas M, Huke J. Number, shape, and topography of leakage points in acute type I central serous retinopathy. Graefes Arch Clin Exp Ophthalmol 1987;225(6):437-40.
8. Haimovici R, Koh S, Gagnon DR, et al. Central Serous Chorioretinopathy Case-Control Study Group. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology 2004 Feb;111(2):244-9.
9. Levy J, Marcus M, Belfair N, et al. Central serous chorioretinopathy in patients receiving systemic corticosteroid therapy. Can J Ophthalmol 2005 Apr;40(2):217-21.
10. Mondal LK, Sarkar K, Datta H, Chatterjee PR. Acute bilateral central serous chorioretinopathy following intra-articular injection of corticosteroid. Indian J Ophthalmol 2005 Jun;53(2):132-4.
11. Haimovici R, Gragoudas ES, Duker JS, et al. Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology 1997 Oct;104(10):1653-60.
12. Bouzas EA, Scott MH, Mastorakos G, et al. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol 1993 Sep;111(9):1229-33.
13. Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology 2003 Apr;110(4):698-703.
14. Fraunfelder FW, Fraunfelder FT. Central serous chorioretinopathy associated with sildenafil. Retina 2008 Apr;28(4):606-9.
15. Kloos P, Laube I, Thoelen A. Obstructive sleep apnea in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2008 Sep;246(9):1225-8.
16. Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Med Hypotheses 2004;63(3):524-7.
17. Hussain D, Gass JD. Idiopathic central serous chorioretinopathy. Indian J Ophthalmol 1998 Sep;46(3):131-7.
18. Marcuson J, Riley T. Central serous chorioretinopathy. Optometry 2008 May;79(5):241-51.
19. Bujarborua D, Chatterjee S, Choudhury A, et al. Fluorescein angiographic features of asymptomatic eyes in central serous chorioretinopathy. Retina 2005 Jun;25(4):422-9.
20. Loo RH, Scott IU, Flynn HW Jr, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 2002 Feb;22(1):19-24.
21. Al-Mujaini A, Wali U, Ganesh A, Montana C. Natural course of central serous chorioretinopathy without subretinal exudates in normal pregnancy. Can J Ophthalmol 2008;43(5):588-90.
22. Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol 1983 Apr;95(4):457-66.
23. Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 2008 Dec;115(12):2229-34.
24. Tarantola RM, Law JC, Recchia FM, et al. Photodynamic therapy as treatment of chronic idiopathic central serous chorioretinopathy. Lasers Surg Med 2008 Dec;40(10):671-5.
25. Shukla D, Kolluru C, Vignesh TP, et al. Transpupillary thermotherapy for subfoveal leaks in central serous chorioretinopathy. Eye 2008 Jan;22(1):100-6.
26. Torres-Soriano ME, Garca-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol 2008 Sep;246(9):1235-9.
27. Meyerle CB, Freund KB, Bhatnagar P, et al. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina 2007 Sep;27(7):943-6.
28. Nielsen JS, Weinreb RN, Yannuzzi L, Jampol LM. Mifepristone treatment of chronic central serous chorioretinopathy. Retina 2007 Jan;27(1):119-22.

