A 45-year-old Hispanic female was referred by her O.D. for a consultation for possible hydroxychloroquine retinopathy/maculopathy. Her chief complaint: metamorphopsia nasal to fixation in the left eye. Previous exam data revealed macular mottling in both eyes, a tritanomolous color vision defect in the right eye and a deuteranomolous color vision defect in the left, possibly secondary to hydroxychloroquine use. A panel D-15 test was noted at the patients last exam with her O.D., which was two weeks prior.
We suspect this patient was referred to us with metamorphopsia and color vision defects because her test results were suspect. This may have resulted because it was her first time taking these tests and/or because her exclusive use of the Spanish language may have handicapped her ability to understand the test instructions.
The patient reported being diagnosed with systemic lupus erythematosus (SLE) two years prior and secondary kidney and liver dysfunction. She said she was treated with 200mg to 400mg of Plaquenil (hydroxychloroquine sulfate, Sanofi-Synthelabo) per day for her lupus (4.4mg/kg to 8.8 mg/kg per day) for two years with a cumulative dose of 276g. The patient said she was also prescribed prednisone for her lupus; the dose had been tapered from 5mg/day to 1mg/day.
She reported seeing her rheumatologist every six months for her lupus, and she reported having blood taken every three months to monitor her kidney and liver function, which were normal. Her family ocular history was unremarkable, though her family medical history included a daughter diagnosed with SLE. Finally, the patient reported an allergy to folic acid.
Diagnostic Data
Her entering corrected visual acuity at distance and near was 20/20 O.U. External exam demonstrated normal motilities, confrontation fields, color vision by D-15 and no pupillary defect. Refraction uncovered negligible changes in her hyperopic prescription.
Biomicroscopy revealed corneal verticillata of the mid-peripheral to inferior cornea O.S. Intraocular pressure measured 12mm Hg O.D. and 14mm Hg O.S. using Goldmann applanation tonometry. Dilated fundus exam revealed cup-to-disc ratios of 0.35 x 0.35 O.D. and 0.40 x 0.40 O.S., with distinct rims and disc margins.
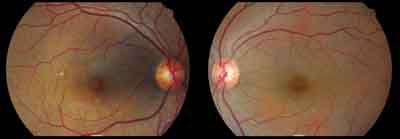 |
| Color fundus photos show a 45-year-old Hispanic female who presented with possible hydroxychloroquine retinopathy/maculopathy. |
Retinal pigment epithelial (RPE) mottling in and around the macula of each eye was observed. A 1.5DD (disc diameter) area of RPE drop-out was noted slightly superior temporal from the macula O.D. Ocular coherence tomography (OCT) revealed what we interpreted as an old nonchoroidal non-neovascularized RPE detachment superior nasal from the right macula. The left eye appeared unremarkable.
A Humphrey SITA-Standard 10-2 and 24-2 visual field showed bilateral defects in the inferior nasal quadrant near fixation, which could be consistent with Plaquenil retinopathy.
Macular photo stress testing was normal with acuity recovery in less than 20 seconds in each eye (<30 seconds is normal, 30 to 60 seconds is suspicious, and >60 seconds is considered abnormal).
Diagnosis
The initial diagnosis was corneal verticillata O.S. and suspected hydroxychloroquine maculopathy O.U. The old RPE detachment was suspected to be secondary to chronic steroid use.18,19
Treatment and Follow-Up
We monitored the conditions and educated the patient on the ocular side effects of hydroxychloroquine and steroid use. She was then given an Amsler grid for self-monitoring, asked to return to the clinic if she noted visual distortions or if her medication dosage changed, and asked to return to the referring clinic in one month for a vision exam, to perform Amsler grid and to repeat the dilated fundus exam. These findings were reported to her rheumatologist, who discontinued the steroids and planned to discontinue the hydroxychloroquine if needed.
Discussion
Plaquenil and Aralen (chloroquine phosphate, Sanofi-Synthelabo) were developed to treat malaria, though they now treat nonmalarial diseases such as rheumatoid arthritis, SLE, and cutaneous lupus.1,2 Hydroxychloroquine, an analogue of chloroquine, is favored over chloroquine because it is better tolerated by patients and is considered far less toxic, as it does not cross the blood-retinal barrier as chloroquine does.3,4
Hydroxychloroquine retinopathy is characterized by a bulls eye maculopathy and is associated with corneal verticillata.2,5,6 The reported incidence of toxic retinopathy associated with chloroquine varies from 1% to 16%; there is a lesser incidence associated with hydroxychloroquine.6,8 The exact mechanism of action of hydroxychloroquine remains uncertain. However, the drug can cause intraepithelial corneal deposits ranging from fine diffuse white, yellow or golden brown punctate to radial or whorl-like lines that converge below the horizontal midline (corneal verticillata); these lines are typically bilateral.7,8 The manifestation of the deposits is not related to drug dosage or duration and may present as early as two to three weeks after the start of treatment. This represents drug retention.8,9
Mild keratopathy is noted in 95% of patients taking 250mg of chloroquine daily, though it is rarely seen in patients taking 400mg of hydroxychloroquine.6,8 Keratopathy may be accompanied by symptoms of halos and is reversible upon discontinuation of both drugs.6,8
Because hydroxychloroquine is a melanotropic drug, it has a high affinity for pigmented eye structures, such as the iris, choroid and retinal pigment epithelium. The drug exerts its toxic effect on the RPE and directly on the photoreceptors. Its slow excretion rate from the body and its high level of accumulation within the eye may account for reports of progressive and delayed retinopathy despite discontinuation of the drug.
Prior to any retinal changes or symptoms, the earliest signs of hydroxychloroquine retinopathy are bilateral relative paracentral scotomas 4 to 9 off central fixation and detectable with Amsler grid. These defects can be confirmed by automated perimetry.2,4,10,11
The earliest observable fundus findings include parafoveal RPE irregularities, including mild stippling or mottling of the macula and reversible loss of foveal reflex.2,6,8,12 The clinical end stage picture of hydroxychloroquine retinopathy: the classic bilateral bulls-eye maculopathy sparing a small foveal island; asymmetry of presentation is not uncommon.8 This stage represents advanced, irreversible damage to the retina with significant irreversible visual field loss.8
With continued drug exposure, extensive ocular damage may occur, including widespread retinal atrophy, leading to vascular narrowing and pigmentary changes (bone spicules) in the peripheral retina and choroid resembling retinitis pigmentosa.8,9 Unilateral symptoms or fundus changes generally are not considered sufficient to im-plicate drug toxicity.9
Drug toxicity is dose and duration related. Hydroxychloroquine is typically prescribed at 200mg/day to 400 mg/day. Dosage should be based on ideal body weight, not actual body weight because the medication is not retained in fatty tissues.3,4,9 A 200mg tablet is safe for all but the smallest patients. A 400mg tablet puts lean or ideal body weight patients of less than 135 pounds in the higher risk category for drug toxicity.9
Because drug clearance occurs renally and hepatically, consider in-sufficiency of these organs to prevent overdosage.1,6,8,13 Overdosing is a problem, especially in obese pa-tients. Recognize this to reduce the incidence of retinal toxicity.3,4
The risk of toxicity is minimal for those patients who:1,2,9,14
Take less than 6.5mg per kilogram of body weight (actual body weight in lean individuals, ideal body weight in overweight people).
Use the medication for less than five years.
Maintain normal renal and liver function during this time.
Do not exceed a cumulative dose of 200mg.
Have no pre-existing retinal disease.
|
Baseline Examination for Patients Treated with Plaquenil |
|
If the complete baseline exam is normal and the patient is at low risk for hydroxychloroquine retinopathy, then follow the recommended screening interval: |
Amsler grid testingcapable of detecting paracentral scotomas in observant individuals within 10 of fixationshould be done as a baseline and at home by the patient.8 A Humphrey 10-2 visual field often confirms defects found by the Amsler grid.6 Conduct baseline color vision testing using a desaturated Farnsworth Panel D-15 to detect underlying congenital or acquired color deficiencies. A desaturated Farnsworth Panel D-15 test may recognize subtle tritan (blue-yellow) defects from acquired maculopathies and is the best test to run; however, a tritan defect is not specific for antimalarial toxicity.9
Assess the corneas after dilation to best detect verticillata.2,9 Carefully examine the patients maculae for pigmentary changes that may be later confused with toxicity. Check the peripheral retina for pigmentation or atrophy, and document the retinal vasculature status.9
Optional tests: fundus photography, fluorescein angiography and multifocal electroretinogram (mfERG).9,12 Fundus photography documents pigmentary changes that may be confused with early toxicity. Consider a fluorescein angiography and mfERG to differentiate underlying maculopathy from toxicity or if the patient is at high risk for early or rapid toxicity.9 The mfERG can detect Plaquenil toxicity earlier than visual acuity, color or Amsler grid testing.13,16
|
Criteria of Low and High Risk for Developing Retinopathy | ||
| Low Risk | High Risk | |
| Daily dosage | <6.5mg/kg hydroxychloroquine | >6.5mg/kg hydroxychloroquine |
| <3mg/kg chloroquine | >3mg/kg chloroquine | |
| Duration of use | <5 years | >5 years |
| Cumulative dose | <200g hydroxychloroquine | >200g hydroxychloroquine |
| <100g chloroquine | >100g chloroquine | |
| Body habitus | lean or average body fat level | high body fat level |
| Renal/liver disease | absent | present |
| Pre-existing retinal disease | absent | present |
| Age | <60 years old | >60 years old |
|
Modified from Marmor M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2002;109(7):1377-82. | ||
After a baseline ophthalmic exam confirms the absence of pre-existing retinal pathology, patients may receive hydroxychloroquine at a maximum daily dosage of 6.5mg/ kg of their ideal body weight (or actual weight if lean). These patients can continue to use the drug safely for five years if renal and hepatic function are normal, and the cumulative dosage is less than 200g. However, annual eye exams should be performed on patients using hydroxychloroqine as recommended by the American College of Rheumatology and the Royal College of Ophthalmology. (The American Academy of Ophthalmology recommends eye exams every two to four years for patients ages 40 to 60.) Consider closer monitoring of patients at higher risk (see Criteria of Low and High Risk for Developing Retinopathy, above). Patients should return more frequently if the dosage increases, significant weight loss occurs, Amsler grid changes are noted, or renal or hepatic dysfunction develops (see Examination Frequency for Low Risk Patientsm, below).
| Examination Frequency for Low Risk Patients | |
| Daily Dosage of Plaquenil | Examination Interval |
| 200mg | 12 months |
|
400mg |
9 months |
| 600mg | 6 months |
If you note toxic retinopathy, hydroxychloroquine should be discontinued. However, substitute treatments, such as glucocorticoids, also have the potential for serious systemic side effects.9,17 While the exact mechanism is unclear, glucocorticoids have been implicated in the development of central serous chorioretinopathy.17,18,19 Because hydroxychloroquine has a long half-life, the full effects may not be noticed for three to six months. Reevaluation every three months after suspected toxicity is advised.9
At three-month follow up, this patients visual acuities were the same, but her macular mottling increased, and her macular photostress test recovery time increased to one minute. Also, there were distortions in her Amsler grid testing. As a result, her rheumatologist discontinued the hydroxychloroquine.
Although hydroxychloroquine retinopathy is rare relative to the many patients using this drug, its increased usage should prompt concern, as there is the potential for progressive and permanent vision loss after cessation of the drug.1,3,9 Know when to be concerned about what constitutes a reasonable approach in monitoring a patient using this drug.
Dr. Pate is a clinical associate professor at the University of Houston College of Optometry. He is an attending O.D. in the Ocular Diagnostic/Medical Eye services and in several community clinics. Dr. Tran provided care for this patient as a student at UHCO.
1. Falcone PM, Paolini L, Lou PL. Hydroxychloroquine toxicity despite normal dose therapy. Ann Ophthalmol 1993 Oct ; 25(10):385-8.
2. Weisinger HS, Pesudovs K, Collin HB. Management of pa-tients undergoing hydroxychloroquine (Plaquenil) therapy. Clin Exper Optom 2000 Jan;83(1):32-6.
3. Easterbrook M. Detection and prevention of maculopathy associated with antimalarial agents. Int Ophthalmol Clin 1999 Spring;39(2)49-57.
4. Browning DJ. Hydroxychloroquine and chloroquine retinopathy: screening for drug toxicity. Am J Ophthalmol 2002 May;133 (5):649-56.
5. Shearer RV, Dubois EL. Ocular changes induced by long-term hydroxychloroquine (Plaquenil) therapy. Am J of Ophthalmol 1967 Aug; 64(2):245-52.
6. Roque M. Chloroquine/Hydroxychloroquine Toxicity. Available at www.emedicine.com/oph/topic245.htm (Accessed Aug- ust 24, 2006)
7. Silman A, Shipley M. Ophthalmological monitoring for hy-droxychloroquine toxicity: a scientific review of available data. Br J Rheumatol 1997 May; 36(5):599-601.
8. Aylward JM. Hydroxychloroquine and chloroquine: assessing the risk of retinal toxicity. J Am Optom Assoc. 1993 Nov;64(11): 787-97.
9. Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: A report by the American Academy of Ophthalmology 2002 Jul; 109(7):1377-82.
10. Blyth C, Lane C. Hydroxychloroquine retinopathy: is screening necessary? BMJ 1998 Mar 7;316(7133):716-7.
11. Penrose RJ, Tzekov RT, Sutter EE, et al. Multifocal electro-retinography evaluation for early detection of retinal dysfunction in patients taking hydroxychloroquine. Retina 2003 Aug;23(4): 503-12.
12. Neubauer AS, Samari-Kermani K, Schaller U, et al. Detecting chloroquine retinopathy: Electrooculogram versus colour vision. Br J Ophthalmol. 2003 Jul;87(7):902-8.
13. Maturi Rk, Minzhou Y, Weleber RG. Multifocal electroretinographic evaluation of long-term hydroxychloroquine users. Arch Ophthalmol 2004 Jul;122(7):973-81.
14. Grierson DJ. Hydroxychloroquine and visual screening in a rheumatology outpatient clinic. Ann Rheum Dis 1997 Mar;56 (3):188-90.
15. Coyle JT. Hydroxychloroquine retinopathy. Ophthalmology 2001 Feb;108(2):243-4.
16. Neubauer AS, Stiefelmeyer S, Berninger T, et al. The multifocal pattern electroretinogram in chloroquine retinopathy. Ophthalmic Res 2004 Mar-Apr;36(2):106-13.
17. Kimberly RP. Prospects for autoimmune disease: Research advances in systemic lupus erythematosus. JAMA 2001 Feb 7;285(5):650-2.
18. Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids Surv Ophthalmol 2002 Sep-Oct;47(5):431-48.
19. Koyama M, Mizota A, Igarashi Y, et al. Seventeen cases of central serous chorioretinopathy associated with systemic corticosteroid therapy. Ophthalmologica.2004 Mar-Apr;218(2):107-10.
20. Mills PV, Beck M, Power BJ. Assessment of the retinal toxicity of hydroxychloroquine. Trans Ophthalmol Soc U K.1981;101 (1):109-13.

