 In our September column, “
Double Trouble,” we described a patient who was being managed for primary open-angle glaucoma (POAG) and subsequently developed double vision from coincident cranial nerve (CN) VI palsy. This month, we discuss yet another patient with glaucoma who developed double vision from a more sinister cause.
In our September column, “
Double Trouble,” we described a patient who was being managed for primary open-angle glaucoma (POAG) and subsequently developed double vision from coincident cranial nerve (CN) VI palsy. This month, we discuss yet another patient with glaucoma who developed double vision from a more sinister cause.
The patient is a 61-year-old black male being managed for POAG who developed a severe headache on a Friday, which he described as “right sided” and a “nine out of 10” in terms of pain and discomfort severity.
At nearly the same time as the onset of his headache, his right eyelid began to droop and he developed double vision. He took over-the-counter analgesics throughout the weekend and, when the debilitating pain did not subside, he came into the office urgently on Monday morning.
Evaluation revealed a well-nourished man in acute distress with a near complete right-sided ptosis. Upon manually elevating the lid, the right eye had assumed a “down-and-out” position. He had significant ophthalmoplegia with an adduction, elevation and depression deficit. Abduction was normal in the right eye and motility was normal in the left. Tellingly, his right pupil was mid-dilated and minimally reactive to light, while the left pupil was smaller and normally reactive.
Clearly, the patient had a right CN III palsy. The dilated right pupil and severe hemicranial pain indicated that the likely cause was an intracranial aneurysm, which was compressing the nerve and pupillomotor fibers. In this month’s column, we discuss one of the only true ophthalmic emergencies: aneurysmal CN III palsy.
What is CN III Palsy?
A patient with acute CN III palsy usually presents with a sudden onset of unilateral ptosis and ophthalmoplegia, which is frequently accompanied by significant eye or head pain––depending on the underlying cause.1-4 Such patients often complain of double vision. But, in some instances, the diplopia may be masked by the ptosis, which obscures the vision in the affected eye; however, when the lid is manually elevated, the patient will experience diplopia. Acuity typically is unaffected unless the provoking lesion occurs in the superior orbital fissure, causing simultaneous CN II involvement.
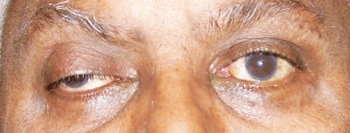
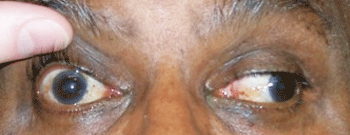
Our patient in primary gaze with right ptosis and an eye that appears down and out (top). In left gaze, he demonstrates an inability to adduct; anisocoria also is evident.
CN III palsy produces a non-comitant exotropic, hypotropic eye position (down and out). There is limitation of elevation, depression and adduction as well as an underaction of the superior, inferior and medial recti muscles and the inferior oblique muscle.1-3 The underaction of these muscles may be complete or incomplete.5-7 In any case of CN III palsy, the pupil may be dilated and minimally reactive to light (pupillary involvement), totally reactive and normal (pupillary non-involvement) or sluggishly responsive (partial pupillary involvement).3,4,7-10
Various neurological signs—such as contralateral intention tremor, cerebellar ataxia or contralateral hemiplegia—may present concomitantly with the development of CN III palsy, depending on the cause and location of damage to CN III within the brainstem.3
Patients who develop acute CN III palsy tend to be older (>55 years of age). CN III palsy is uncommon in children, although it can occur.7 Often, there is concurrent diabetes and/or hypertension in older adults.3,6,7,11 Occasionally, head trauma is associated with the development of CN III palsy.12
Third nerve palsy results from damage to the oculomotor nerve anywhere along its route from the nucleus in the dorsal mesencephalon, its fascicles in the brainstem parenchyma, the nerve root in subarachnoid space, the cavernous sinus or the posterior orbit.3,13
The main concern in an isolated CN III palsy occurring within the subarachnoid space is nerve compression caused by an expanding aneurysm of the posterior communicating artery (most common) or the internal carotid, basilar, anterior communicating or temporal arteries (less common).8,9,14-16
Approximately 15% of isolated CN III palsies occurring secondary to damage within the subarachnoid area are due to aneurysms.11 Vasculopathic infarct, often associated with concurrent diabetes or hypertension, accounts for 35% of isolated CN III palsy cases.11
Aneursymal compression is marked by head or retro-orbital pain and anisocoria with ipsilateral pupil dilation, because the expanding aneurysm compresses the pupillomotor fibers traveling with CN III as well as pain-sensitive dura and other such structures. Patients who develop CN III palsy from aneurysmal compression may not present initially with anisocoria or pupil involvement.5,8-10 Instead, these patients typically present with an incomplete palsy that evolves and develops pupil dilation over several days.3,7, 11
Managing CN III Palsy
Management of CN III palsy in adults depends on the associated findings and etiology. In complicated CN III palsies––where other neural structures are involved––the patient should undergo MRI scanning in order to ascertain the etiology.3
In cases of isolated, complete CN III palsies that have no pupillary involvement in patients over 50 years of age, the primary cause is typically ischemic vascular infarct. Giant cell arteritis also is a potential etiology. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA), erythrocyte sedimentation rate, C-reactive protein, blood pressure measurement, complete blood count with differential, and blood glucose testing are indicated. CT scan and CT angiography (CTA) are often used to identify intracranial bleeding as well as identify the aneurysm location.
Close observation is required, because pupil involvement may be delayed by five to seven days. This is especially true for patients with incomplete CN III palsy with pupil sparing, because they are more likely to develop an incipient aneurysm.5
In ischemic vascular CN III palsy, the pupil will not evolve, aberrant regeneration will not occur, and the palsy will spontaneously improve or resolve over the course of three to six months.3,11 If the palsy shows no improvement over six to eight weeks or aberrant regeneration develops, MRI/MRA or CT/CTA is required to rule out the presence of an occult mass in the subarachnoid space.3
If the patient is less than 50 years of age and has an isolated, non-pupillary-involved CN III palsy, imaging or intracranial angiography is indicated. In this age group, ischemic vasculopathy is less likely to occur than an aneurysm.
If an adult patient of any age presents with isolated, complete or incomplete CN III palsy with pupillary involvement, this should be considered a medical emergency. The patient should undergo immediate MRA/MRI or intracranial angiography. In these cases, the cause likely is an aneurysm located at the junction of the internal carotid and posterior communicating arteries, or at the tip of the basilar artery. These patients should be sent immediately to a hospital emergency room with the diagnosis and recommendations for consultation.
If the aneurysm ruptures, there is a risk of death from subarachnoid hemorrhage and brainstem herniation through the foramen magnum. In cases of CN III palsy caused by subarachnoid aneurysm, immediate neurosurgical intervention is necessary. Common endovascular treatment involves direct clipping of the aneurysm or embolization with detachable coils.16,17
Pupil-involved CN III palsy in adults is one of the few true medical emergencies seen in eye care. These patients must be sent to the hospital immediately for neurosurgical consult.
In this patient’s case, we immediately suspected an intracranial aneurysm. The patient and his wife were well educated about the potential mortality risk and promptly agreed to go to the hospital emergency room, where a triage nurse had already been alerted and was waiting. The patient’s wife called back 45 minutes later to report that her husband was undergoing neuroimaging and a neurosurgical consult.
Ultimately, he was diagnosed with an internal carotid artery aneurysm and underwent embolization with detachable coils. The patient spent 22 days in the intensive care unit and more than one year later still continues to recover, underscoring the seriousness of this condition.
1. Satyarthee GD, Mahapatra AK. Unusual neuro-ophthalmic presentation of anterior communicating artery aneurysm with third nerve paresis. J Clin Neurosci. 2004 Sep;11(7):776-8.
2. Bahmani Kashkouli M, Khalatbari MR, Yahyavi ST, et al. Pituitary apoplexy presenting as acute painful isolated unilateral third cranial nerve palsy. Arch Iran Med. 2008 Jul;11(4):466-8.
3. Yanovitch T, Buckley E. Diagnosis and management of third nerve palsy. Curr Opin Ophthalmol. 2007;18(5):373-8.
4. Delengocky T, Bui CM. Complete ophthalmoplegia with pupillary involvement as an initial clinical presentation of herpes zoster ophthalmicus. J Am Osteopath Assoc. 2008 Oct;108(10):615-21.
5. Takahashi M, Kase M, Suzuki Y, et al. Incomplete oculomotor palsy with pupil sparing caused by compression of the oculomotor nerve by a posterior communicating posterior cerebral aneurysm. Jpn J Ophthalmol. 2007;51(6):470-3.
6. Capó H, Warren F, Kupersmith MJ. Evolution of oculomotor nerve palsies. J Clin Neuroophthalmol. 1992 Mar;12(1):21-5.
7. Richards BW, Jones FR Jr, Younge BR, et al. Causes and prognosis in 4,278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am J Ophthalmol. 1992;113(5):489-96.
8. Kasoff I, Kelly DL Jr. Pupillary sparing in oculomotor palsy from internal carotid aneurysm. Case report. J Neurosurg. 1975 Jun;42(6):713-7.
9. Kissel JT, Burde RM, Klingele TG, et al. Pupil-sparing oculomotor palsies with internal carotid-posterior communicating artery aneurysms. Ann Neurol. 1983 Feb;13(2):149-54.
10. Saito R, Sugawara T, Mikawa S, et al. Pupil-sparing oculomotor nerve paresis as an early symptom of unruptured internal carotid-posterior communicating artery aneurysms: three case reports. Neurol Med Chir (Tokyo). 2008 Jul;48(7):304-6.
11. Akagi T, Miyamoto K, Kashii S, et al. Cause and prognosis of neurologically isolated third, fourth, or sixth cranial nerve dysfunction in cases of oculomotor palsy. Jpn J Ophthalmol. 2008 Jan-Feb;52(1):32-5.
12. Adams ME, Linn J, Yousry I. Pathology of the ocular motor nerves III, IV, and VI. Neuroimaging Clin N Am. 2008 May;18(2):261-82.
13. White JB, Layton KF, Cloft HJ. Isolated third nerve palsy associated with a ruptured anterior communicating artery aneurysm. Neurocrit Care. 2007;7(3):260-2.
14. Aiba T, Fukuda M. Unilateral oculomotor nerve paresis associated with anterior communicating artery aneurysm rupture--two case reports. Neurol Med Chir (Tokyo). 2003 Oct;43(10):484-7.
15. Asakura K, Tasaki T, Okada K. A case of unruptured anterior temporal artery aneurysm showing pupil-sparing oculomotor palsy. No Shinkei Geka. 1986 May;14(6):777-82.
16. Inamasu J, Nakamura Y, Saito R, et al. Early resolution of third nerve palsy following endovascular treatment of a posterior communicating artery aneurysm. J Neuroophthalmol. 2002 Mar;22(1):12-4.
17. Ahn JY, Han IB, Yoon PH, et al. Clipping vs coiling of posterior communicating artery aneurysms with third nerve palsy. Neurology. 2006 Jan;66(1):121-3.

