
Elevated intraocular pressure has long been associated with primary open-angle glaucoma, and all current therapies for this disease involve medical or surgical procedures designed to lower IOP. The effectiveness of this approach has been scientifically validated over the last decade with the publication of results from large-scale randomized clinical trials.1-3
While these findings clearly demonstrate that IOP reduction is beneficial in decreasing progression, treatment does not necessarily prevent disease advancement.1-3 The goal of glaucoma management is to stop the disease from worsening, but every clinician is familiar with the frustration of disease progression despite careful follow-up and judicious use of current therapies.
In the future, will it be possible to do better? Ongoing research gives us reason to be optimistic.
Theres no question that there will be continued improvements in existing diagnostic technology, and that these changes will enable better detection of both structural and functional changes that result from glaucoma-related optic nerve damage. In addition, there is likely to be further optimization of current IOP-lowering therapies, including innovations directed toward increasing patient compliance. There is also the potential for paradigm-shifting breakthroughs in laboratory-based research.
Heres a brief overview of four promising areas of research that, although not yet ready for application in humans, have the potential to dramatically change how glaucoma is treated in the future.
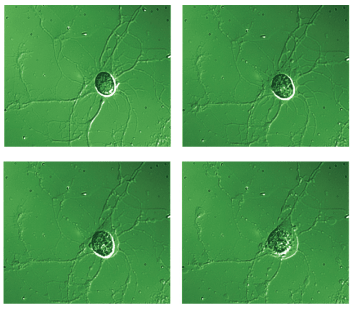
These time-lapsed images record the death of a retinal ganglion cell (RGC), isolated from a rat, during a two-hour exposure to the excitotoxin glutamate. Laboratory studies on isolated RGCs and on retinas from animals with experimental glaucoma have provided details on the cellular and molecular events that occur during RGC death. Neuroprotective strategies aim to interfere with these mechanisms, thereby increasing RGC survival.
Figure from Hartwick, et al. (2008)31 with permission from Wiley-Blackwell Publishing.
Continuous IOP Monitoring
Even though a single IOP value is often recorded in patients charts during each office visit, we must remember that IOP is always changing. Continuous monitoring of IOP has been achieved in laboratory animals (rabbits, rats and mice), and the results from this work indicate that IOP can exhibit considerable variability throughout the animals daily cycle.4-6
In these studies, the tip of a catheter was surgically inserted into the anterior or vitreous chamber and pressure was conducted through the catheter to a battery-operated transducer implanted outside the eye. IOP measurements were then transmitted via a wireless radio signal to a nearby receiver for subsequent computer analysis.
Not only did these studies confirm that there is a circadian rhythm to IOPin which IOP is generally elevated (by roughly 5mm Hg) during the night compared with daytime levelsbut also that the IOP in these freely-moving animals showed significant minute-to-minute and day-to-day fluctuations not readily apparent in periodic IOP measurements obtained by standard tonometry.
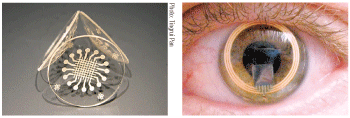
Two examples of experimental contact lenses that continuously monitor IOP: A smart contact lens developed by biomedical engineers at
Right, the Triggerfish lens, developed by Swiss-based Sensimed AG. The IOP data detected by sensors in each lens could be transmitted to a data-storing device.
In humans, IOP variability during certain real-life situations might be quite different than values measured in a sitting patient during an eye exam. Because IOP is the only modifiable risk factor in glaucoma, it would be useful to know a glaucoma patients cumulative IOP exposure as opposed to the snapshots of IOP we use now.
Furthermore, IOP fluctuation may be a strong risk factor on its own for the risk of disease progression in glaucoma patients, independent from mean IOP levels.7 But, findings from the recently published clinical glaucoma trials regarding this association disagree. The Advanced Glaucoma Intervention Study supported IOP fluctuation as a significant risk factor for glaucoma progression, but the Early Manifest Glaucoma Trial did not.8,9
One reason for why IOP fluctuation may be a risk factor is that patients with variable IOP might be more prone to exhibit transient spikes (large increases) in IOP. The peak IOP reached during these spikes could be a better predictor for future glaucoma progression than the daily mean IOP.
To that end, work is in progress to adapt the implantable IOP sensors used in laboratory research for human use by developing smaller, ultra-low-power, biocompatible pressure sensors that could either be implanted (subsclerally or intraocularly) in the eye or incorporated into soft contact lenses.10,11 The IOP detected by the sensor could then be transmitted wirelessly to a data-storing device worn by the patient.
Besides contributing to our overall understanding of IOP fluctuations in glaucoma patients, this data could be used to ensure that a more appropriate target IOP level is chosen for each patient. Specifically, a lower target mean IOP might be set for a patient in which IOP variability is greater, resulting in more individualized therapeutic regimens. Also, the information could be sent electronically to the doctors office on a regular basis, alerting the clinician to periods in between appointments when the IOP stays above the desired target range. In these instances, the clinician can proactively alter the treatment.
Also, the reinforcing feedback provided by the continuous IOP record may serve to increase patient compliance to treatment.
Gene Therapy
Gene therapy involves the transfer and incorporation of new genes into a cells genome.
The eye may be a particularly amenable structure for gene therapy because its relative anatomical isolation lessens the risk that gene insertion will occur in a tissue unaffected by the disease of interest.12 In laboratory animals, genes have been incorporated into various ocular cells following injection of gene-carrying vehicles (known as vectors) into the anterior chamber, vitreous chamber or subretinal space.
Although glaucoma-related gene therapy has yet to reach the clinical trial stage, the results from two investigations involving six human patients with Lebers congenital amaurosis have been reported within the last year.13,14 In these patients, the gene RPE65, responsible for encoding a protein involved in vitamin A recycling in the retina (a necessary component of rod/cone phototransduction), was defective, and a vector containing replacement human RPE65 was injected into the subretinal space.
Remarkably, four of the six patients exhibited modest increases in visual acuity over the first few months following treatment and, although a surgery-related macular hole developed in one patient, other significant adverse effects have yet to be reported.13,14 These exciting early results give credence to the idea that gene therapy holds promise for the future treatment of various retinal disorders.
For glaucoma, a number of known genes associated with open-angle glaucoma (such as myocilin) represent potential targets for gene therapy.15 However, most cases of open-angle glaucoma are likely to be multifactorial, rather than being caused by a single-gene mutation. But, instead of replacing a defective gene, gene transfer techniques could be used to reprogram cells so that they overproduce a therapeutically-useful compound.
One strategy would be to target retinal cells so that survival factors could be released in the vicinity of retinal ganglion cells (RGCs).
Another strategy is to increase aqueous outflow (thereby reducing IOP) in the anterior chamber by transferring genes to cells in the trabecular meshwork (TM).16 TM cells possess contractile properties that can be affected by certain cytoskeleton regulatory proteins. Using vectors, genes for these proteins have been transferred to TM cells in cultured monkey anterior segments.17,18 The resulting over-expression of these proteins led to changes in the TM cytoskeletal architecture accompanied by an increase in aqueous outflow facility.
Future work will focus on determining whether this method can increase outflow in living animals and the extent to which IOP can be decreased using this technique. Researchers will also need to understand the length of time the effect can persist following a single injection.
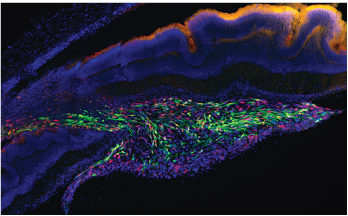
Human retina-derived stem cells (shown in green) were transplanted into the retina of a rat with experimental glaucoma. Some of the transplanted cells differentiated into cells that expressed neuron markers.
Figure from Bull, et al (2008), used with permission.30
Stem Cells
Stem cells have received an incredible amount of media attention during the last few years. The subject has aroused considerable public interest and expectations.
Stem cells can be broadly defined as cells that are capable of self-renewal and differentiating into multiple cell types. Because the neurons that die in neurodegenerative diseases (including glaucoma) are lost forever, stem cells represent a potential mechanism by which the lost neurons could be replaced by new ones.
The theory is that stem cells could be isolated, induced to transform into the neuron or cell of interest, and transplanted into regions where cell replacement is desired. This would obviously represent a dramatic shift in the treatment of any neurodegenerative condition; in addition to preventing future neuronal death, functional deficits associated with the condition (i.e., vision loss in glaucoma) could be reversed.
Vision researchers are also at the forefront of advances in stem cell-based therapies. Recently, investigators showed in mice that if retinal progenitor cells (cells that later transform into the various retinal cell types) are isolated at the stage of development associated with rod formation, these cells can differentiate into rods, make appropriate retinal connections and contribute to visual function after their transplantation into the retinas of adult mice.19 This provided proof-of-principle for cell replacement as a viable option for retinal repair, but its application to humans is currently complicated by the ethical questions associated with obtaining cells from embryonic tissue.
A line of future inquiry will focus on coaxing stem cells from other sources to transform into the desired cell type for subsequent retinal transplantation. One intriguing example: Stem cells found in the adult human ciliary epithelium may be used to integrate into host retinas.20,21 To achieve maximal vision restoration in glaucoma patients, the ideal replacement cell would be stem cell-derived RGCs because it is these retinal output neurons that are primarily lost in POAG.
This adds inherent complications not associated with photoreceptor replacement strategies. Not only would the new RGCs have to functionally integrate themselves into the existing retinal circuitry, but the axons of these cells would have to find their way to the optic nerve head, travel a relatively long distance within the optic nerve, and make functional connections with the appropriate target neurons in the brain.
This feat may even have additional difficulties, as there is evidence that the target neurons (in the lateral geniculate nucleus, for example) also degenerate in glaucoma patients following RGC loss.22
As an alternative to RGC replacement, researchers are looking at using stem cells as a means to get therapeutic compounds delivered to the retina.23 In this approach, the goal is to stop or slow down further neuronal degeneration, a concept known as neuroprotection.
Neuroprotection
Although IOP-lowering compounds are neuroprotective in a sense, this term is usually reserved for compounds that are designed to more directly interfere with the process of RGC death that occurs in glaucoma. A neuroprotective agent for glaucoma would likely be used in conjunction with IOP-lowering therapies and, ideally, would stop the delayed RGC death that is thought to continue even after IOP is reduced.
There has been much accomplished in understanding the cellular and molecular events that occur as an RGC dies. This information has been gleaned from studies of isolated RGCs exposed to various insults and from studies of animals with experimental glaucoma (elevated IOP), and numerous targets for therapeutic intervention have been identified.24-26
However, while interest in neuroprotective treatment strategies for glaucoma began 10 to 15 years ago, a proven neuroprotective drug for glaucoma is not yet clinically available. There have been a number of obstacles slowing down this research, not the least of which is getting consistent and chronic delivery of the potential therapeutic agent to the retina or optic nerve.
Non-conventional methods of drug delivery that utilize gene transfer techniques or stem cell transplantation are currently being explored. For example, there is evidence that the flow of neurotrophins that occurs within RGC axons is disrupted at the optic nerve head during open angle glaucoma, and that the ensuing neurotrophin deprivation is a key trigger of glaucomatous RGC death.27
While neurotrophin supplementation is known to protect against RGC death in various experimental models, it is challenging to chronically increase neurotrophin levels in the retinas of living animals. The induction of retinal over-expression of one neurotrophin (brain-derived neurotrophic factor, BDNF), by intravitreally injecting a viral vector containing the BDNF gene, reduced RGC death in rats with experimentally-elevated IOP.28
Similarly, researchers have shown that it is possible to increase neurotrophin levels in rat retinas through stem cell transplantation and that this enhances RGC survival in eyes subjected to ocular hypertension.29
When it comes to predicting which ideas will eventually achieve widespread clinical application, the crystal ball is admittedly murky. Nevertheless, as the preceding examples illustrate, there is hope that continued vision loss in glaucoma patients will one day become a thing of the past.
Dr. Hartwick is an assistant professor at The Ohio State University College of Optometry where he teaches the Monocular Sensory Processes course and conducts research primarily focused on retinal ganglion cell neurobiology.
1. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressure. Am J Ophthalmol 1998 Oct;126(4):487-97.
2. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypertensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002 Jun;120(6):701-13.
3. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002 Oct;120(10):1268-79.
4. McLaren JW, Brubaker RF, FitzSimon JS. Continuous measurement of intraocular pressure in rabbits by telemetry. Invest Ophthalmol Vis Sci 1996 May;37(6):966-75.
5. Li R, Liu JHK. Telemetric monitoring of 24 h intraocular pressure in conscious and freely moving C57BL/6J and CBA/CaJ mice. Mol Vis 2008 Apr 18;14:745-9.
6. Valderrama CM, Li R, Liu JHK. Direct effect of light on 24-h variation of aqueous humor protein concentration in Sprague-Dawley rats. Exp Eye Res 2008 Nov;87(5):487-91.
7. Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000 Apr;9(2):134-42.
8. Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004 Sep;111(9):1627-35.
9. Bengtsson B, Leske MC, Hyman L, et al. Fluctuation of intraocular pressure and glaucoma progression in the Early Manifest Glaucoma Trial. Ophthalmology 2007 Feb;114(2):205-9.
10. Dresher RP, Irazoqui PP. A compact nanopower low output impedance CMOS operational amplifier for wireless intraocular pressure recordings. Conf Proc IEEE Eng Med Biol Soc 2007;2007:6056-9.
11. Leonardi M, Pitchon EM, Bertsch A, et al. Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmologica 2008 Nov 11 [Epub ahead of print].
12. Martin KR, Quigley HA. Gene therapy for optic nerve disease. Eye 2004 Nov;18(11):1049-55.
13. Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Lebers congenital amaurosis. N Engl J Med 2008 May 22;358(21):2231-9.
14. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Lebers congenital amaurosis. N Engl J Med 2008 May 22;358(21):2240-8.
15. Borras T, Brandt CR, Nickells R, Ritch R. Gene therapy for glaucoma: treating a multifaceted chronic disease. Invest Ophthalmol Vis Sci 2002 Aug;43(8):2513-8.
16. Kaufman PL. Enhancing trabecular outflow by disrupting the actin cytoskeleton, increasing uveoscleral outflow with prostaglandins, and understanding the pathophysiology of presbyopia. Exp Eye Res 2008 Jan;86(1):3-17.
17. Liu X, Hu Y, Filla MS, et al. The effects of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis 2005 Dec 13;11:1112-21.
18. Gabelt BT, Hu Y, Vittitow JL, et al. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp Eye Res 2006 Jun;82(6):935-44.
19. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature 2006 Nov 9;444(7116):203-7.
20. Tropepe V, Coles BL, Chiasson BJ et al. Retinal stem cells in the adult mammalian eye. Science 2000 Mar 17;287(5460):2032-6.
21. Coles BL, Angenieux B, Inoue T, et al. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci USA 2004 Nov 2;101(44):15772-7.
22. Yucel Y, Gupta N. Glaucoma of the brain: a disease model for the study of transsynaptic neural degeneration. Prog Brain Res 2008;173:465-78.
23. Bull ND, Johnson TV, Martin KR. Stem cells for neuroprotection in glaucoma. Prog Brain Res 2008;173:511-9.
24. Hartwick AT. Beyond intraocular pressure: neuroprotective strategies for future glaucoma therapy. Optom Vis Sci 2001 Feb;78(2):85-94.
25. Cheung W, Guo L, Cordeiro MF. Neuroprotection in glaucoma: drug-based approaches. Optom Vis Sci 2008 Jun;85(6):406-16.
26. Lebrun-Julien F, Di Polo A. Molecular and cell-based approaches for neuroprotection in glaucoma. Optom Vis Sci 2008 Jun;85(6):417-24.
27. Quigley HA. New paradigms in the mechanisms and management of glaucoma. Eye 2005 Dec;19(12):1241-8.
28. Martin KR, Quigley HA, Zack DJ, et al. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci 2003 Oct;44(10):4357-65.
29. Yu S, Tanabe T, Dezawa M, et al. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem Biophys Res Commun 2006 Jun 16;344(4):1071-9.
30. Bull ND,
31. Hartwick AT, Hamilton CM, Baldridge WH. Glutamatergic calcium dynamics and deregulation of rat retinal ganglion cells. J Physiol 2008 Jul 15;586(14):3425-46.

