There’s more than one way to skin a cat. This famous, 17th century English proverb is applicable to just about every experience, encounter and undertaking in life––including eye care. While certain medico-legal guidelines must be followed, effective patient management can be achieved through different avenues.
Here, Dr. Gerson, who is in private practice, and Dr. Shechtman, who is an associate professor of optometry at an academic institution, discuss how they would treat and manage patients who present with diabetic retinopathy (DR), age-related macular degeneration (AMD) and retinitis pigmentosa (RP). Is one approach more appropriate than the other, or are they both equally effective treatment strategies? You decide.
Case 1: Moderate DR
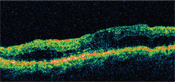
Optical coherence tomography often aids in the evaluation of diabetic macular edema, as seen in this patient.
A 54-year-old white male presented with moderate diabetic retinopathy and high blood pressure. He has not seen a primary care physician in more than a year.
Dr. Gerson: When speaking to patients with diabetes, I always ask them about their hemoglobin A1c. This is because study after study, such as the Diabetes Control and Complications Trial (DCCT) and the UK Prospective Diabetes Study (UKPDS), has shown us how important it is to keep this marker in control.1,2 Ideally, patients should know what the test is, when they had it done and the result. But, the sad truth is that many don’t know what the test is and aren’t having it performed frequently enough.
In this particular case, it is obvious that the patient hasn’t undergone HbA1c testing in at least a year. I would schedule this patient for an in-office blood test immediately. Also, I would send a letter to this patient’s primary care physician and endocrinologist to let them know what testing I have performed and the respective results.
Being able to attain a lab result at the patient’s visit is more powerful and influential than making him or her wait to hear the outcome. We not only need to care for our patients’ eyes, but also their overall well-being. If a patient with diabetes does effectively control his or her HbA1c, it will eventually affect his or her eyes, as has already happened in this case.
In order to perform an in-office HbA1c test, you must have a Clinical Laboratory Improvement Amendments (CLIA) waiver. CLIA sets standards and issues certificates for clinical lab testing. It is fairly easy to obtain the waiver, which requires a form and a nominal fee.
Of course, you will also need a “sharps” container for any waste from the blood draw. The HbA1c tests I use come in packs of 10. Bill for the test with CPT code 83037; the allowable amount is approximately $15. Although this will not be a profit center for your office, it is an excellent service to provide your patients, and it sets your practice apart.
Dr. Shechtman: Interaction between hypertension and diabetes leads to various vascular-related complications, and optometrists are essential to the management of these patients. This goes beyond screening for the spectrum of ocular manifestations associated with both diseases.
So, in addition to fasting blood sugar and HbA1c testing, I often take blood pressure in-office. This simple test reaffirms my role as a primary care physician. In many cases, it helps determine urgency vs. emergency, and it can identify undiagnosed cases of hypertension and those patients who are potentially under-treated.
I agree with Dr. Gerson––we need to counsel our patients about hypertension and diabetes, and how these diseases interact with each other, the patient’s eyes and his or her other organs. Also, we need to individually address our patients’ questions and concerns when designing a personalized management plan. This increases patient compliance and decreases the risk for associated complications. The DCCT/Epidemiology of Diabetes Interventions and Complications Study (EDIC) results showed that patients who exhibited tighter control over their diabetes experienced a decrease in associated diabetic-related complications compared to patients who merely followed a conventional care protocol.1
You must take an interdisciplinary approach when managing patients with vascular conditions. I send summary reports of all ocular findings to each doctor participating in a patient’s management plan. And, and in this particular case, I would encourage the patient to see his primary care physician. Doctors who participate in the care of the diabetic patient may include a primary care physician, a cardiologist and an endocrinologist. Also, these continuous summary reports often result in future referrals to my office.
Dr. Gerson: Regarding management, this patient likely needs to be seen by an eye care provider at least twice a year. I will follow a patient until it is clear that he or she requires intervention that I cannot provide. If the patient does not have clinically significant macular edema (CSME) as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS), or proliferative DR, I will likely continue to follow him or her.3
Additionally, both fundus photography and optical coherence tomography are useful in monitoring these patients. Although ETDRS defines CSME by means of clinical examination, OCT is effective, though not necessary, for following mild changes in macular thickness. When you are recording such changes in macular thickness, OCT is a billable procedure, which generally reimburses between $35 and $40 per tested eye. Although baseline photography is a sensible consideration, I don’t necessarily see the need to do it, because referral for treatment isn’t based so much on changes in fundus appearance as the actual findings. That said, fundus photography could play a fundamental role in your patients’ education. If they can see the changes in their eyes and contrast them with healthy eyes, it might help motivate them to be more compliant with general diabetes control.
Dr. Shechtman: Continuous monitoring of this patient is a vital aspect of his management plan. Yet, I often ask patients with DR who also have high blood pressure to return for follow-up earlier than the ETDRS guidelines mandate, because these patients have an increased likelihood of DR progression or additional complications.2
In my academic setting, I have access to several diagnostic modalities that help facilitate evaluation of DR. These include, but are not limited to, fundus photography and spectral domain optical coherence tomography (SD-OCT).
Keep in mind that you cannot bill for an OCT and a fundus photo at the same visit. Even though CSME is a clinical diagnosis, OCT often aids in the evaluation of macular edema and can also help uncover other associated complications, such as neurosensory retinal detachment or vitreomacular traction.
It is important to remember that CSME can occur at any stage of DR and is not necessarily associated with decreased visual acuity. If I determined that CSME is present, I would refer this patient to a retinal specialist.
In general, it is best to refer such a patient directly to a retinal specialist as to not delay appropriate treatment intervention. Yet, I understand that certain insurance-related limitations might exist for some patients. In that case, a referral to a general ophthalmologist would be necessary.
Case 2: Mild AMD
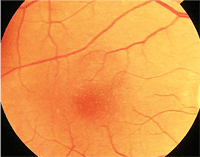
Will this patient with mild AMD benefit from a lutein supplement?
A 68-year-old white male who smokes presented with mild age-related macular degeneration.
Dr. Shechtman: With the number of Americans affected by AMD expected to increase, we need to take a proactive approach in educating our patients about lifestyle changes, smoking cessation and the protective benefits of proper nutrition and nutritional supplements. In addition, we need to educate AMD patients about the benefits of both UV protection and the various lens tints available. The bottom line: Education and counseling is a crucial aspect of managing any patient with AMD and some aspects are covered under the Medicare Physician Quality Reporting Initiative (PQRI).
As we all know, healthy eyes come from healthy bodies, so overall wellness counseling is critical to prevent or slow the progression of AMD. Yet, we must be realistic. Very few patients actually follow the four basic guidelines for overall health: don’t smoke; maintain an adequate body mass index; eat more than five fruits and/or vegetables a day; and exercise 30 minutes per day. So, individual supplementation may be a necessity.
Our patients are faced with an array of supplements at retail stores, making nutritional consultation even more important. When considering the proper nutritional supplementation, you must decide which antioxidants are best for your individual patients. Ask yourself questions such as: When should the patient consider lutein and zeaxanthin, and what are their benefits? How much lutein and zeaxanthin is “adequate” or “safe?” What are the benefits of omega-3 fatty acids? How much omega-3 should I recommend? Are there any complications associated with omega-3s? What function do other vitamins provide in the management of AMD? The answers to most of these questions will vary for each patient.
Dr. Gerson: For patients with AMD, it is important to try to eliminate any modifiable risk factors. Like Dr. Shectman, I focus on nutrition, smoking history, exercise routine and body mass index. Now, I don’t weigh or measure my patients, but I can get a pretty good idea of whether they are overweight just by looking. I ask people about the types of foods they eat. I don’t just ask if they eat vegetables and fruit, but what vegetables and fruit as well as the weekly intake volume. There is a big difference between eating french fries (which account for about 25% of American “vegetable” intake) and a spinach salad with orange peppers on it.
Omega-3 fatty acids have been shown to be beneficial in combating AMD development.4 So, I also ask about fish consumption and whether it is fried. If patients aren’t fish eaters, I generally recommend omega-3 supplementation.
Further, the overall type of foods eaten is important with respect to glycemic index. It is essential to try to eat foods with lower glycemic index, as this has also been shown to be a risk factor for AMD.5
In regard to smoking, numerous studies have conclusively demonstrated that it is bad to smoke if you have AMD.6,7 In summary, people who smoke have worse overall outcomes. I make certain that my patients know this, and often discuss the availability of prescription medications that might help facilitate cessation.
I always inform my patients that high BMI is a risk factor for AMD as well.8 So, I extensively discuss the importance of exercise and good general physical condition.
These rudimentary recommendations are critical to both ocular and general systemic health. So, as Dr. Shechtman suggested, when you recommend such lifestyle alterations, you are not only improving outcomes for AMD, but also your patients’ overall wellness.
Dr. Shechtman: Dr. Gerson raises a very good point about the benefits of proper dietary intake. Various studies have provided guidance regarding the recommendation of nutritional supplements for the management of AMD. Clearly, the largest study to date was documented in AREDS.9 But, it is important to make individual decisions on the proper recommendations of supplements, and an AREDS-formulated vitamin might not be the proper nutritional supplement for all AMD patients.
In the case presented, I would likely recommend a formula with levels of antioxidants, lutein, zeaxanthin and omega-3. This patient might also benefit from a multivitamin for overall general health. However, a few studies have uncovered an increased risk of lung cancer in smokers who receive high doses of beta-carotene.10 So, this patient should avoid formulas with beta-carotene. Currently, AREDS 2 is evaluating the effects of a refined AREDS formula––no beta-carotene, lower levels of zinc, as well as the addition of lutein, zeaxanthin and omega-3. It will be very interesting to see these results several years from now.
Dr. Gerson: Some O.D.s might advocate an AREDS-formulated vitamin for this patient. However, AREDS did not show that patients with mild AMD benefit from nutritional supplementation.9 This finding is likely related to the amount of time required to document statistical significance in conversion from mild dry AMD to wet AMD. That aside, I believe that there is too much zinc in AREDS-formulated supplements for most patients.
Because of the potential risk for zinc overdose, I agree with Dr. Shechtman and believe that this patient is much more likely to benefit from lutein and zeaxanthin supplements than an AREDS-formulated vitamin.
Several studies, including the Lutein Antioxidant Supplementation Trial (LAST), have shown that lutein and zeaxanthin can actually improve vision and reduce drusen in patients with mild AMD.11,12 In addition, this patient would likely benefit from omega-3 supplementation as well.
Dr. Shechtman: Regarding management strategies, I typically follow-up with my AMD patients every three to six months, depending on the stage of the disease and the presence of associated risk factors. I believe that baseline photography, as well as OCT, helps to track further disease progression. Additional tests may include fundus autofluorescence (FAF), Foresee Preferential Hyperacuity Perimeter (Reichert), and a macular pigment optical density (MPOD) measurement.
Dr. Gerson: Generally, I see my patients with mild AMD at least twice a year. Often, they ask if insurance covers biannual visits, and the answer is “yes.” And, like Dr. Shechtman, I take baseline fundus photographs to help document and track any changes over time.
An Optomap (Optos) image of a 21-year-old patient who presented with what appeared as undiagnosed retinitis pigmentosa.
Case 3: Retinitis Pigmentosa
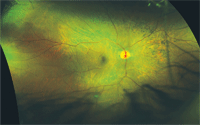
A 21-year-old white female patient presented with what looks like undiagnosed retinitis pigmentosa (RP).
Dr. Gerson: I see patients with congenital dystrophies several times a year. The initial question is whether to send them for electrodiagnostic testing and/or genetic counseling.
I often discuss this with the patient and give him or her the option for more extensive testing. In most cases of congenital dystrophies, the actual name given to the condition doesn’t affect the treatment prescribed. In fact, quite often, the patient won’t receive treatment.
It is important to give patients an idea of the potential visual consequences and lifestyle implications of their condition so that they can plan accordingly. Genetic testing and counseling are important considerations for younger patients with congenital dystrophies because of the potential impact upon their offspring.
For older patients, I recommend that their children and/or grandchildren undergo an eye exam to rule out the presence of congenital dystrophies.
Dr. Shechtman: Although the diagnosis of RP can be made based on clinical presentation, an electroretinogram (ERG) will confirm it. Working at an academic institution, I have access to an electrophysiology clinic. Other ancillary tests may include visual fields and OCT. While the visual field can help monitor for functional disease progression, the OCT helps evaluate associated maculopathies, especially in challenging cases in which there is accompanying extensive retinal degeneration.
Although there is no standard of care for treatment of RP, research has advocated the use of high-dosage vitamin A palmitate therapy.13 More recent studies have recommended that patients with RP who are beginning vitamin A therapy might experience decreased progression of the disease with concurrent docosahexaenoic acid (DHA) supplementation.14 New understanding in the pathophysiology of this disease continues to grow, which will result in new treatment options for patients with RP.
Dr. Gerson: Looking at management protocol, the most important thing is to educate this patient about her condition. It is also important for her to receive routine eye care. Finally, just because this patient has RP does not mean that she won’t develop another ocular condition, such as glaucoma.
Dr. Shechtman: In addition to genetic counseling, as Dr. Gerson mentioned earlier, we should also consider psychological counseling. Patients who face a diagnosis of a degenerative disease, which may cause devastating vision loss, often require psychological counseling for a host of reasons. And, let’s not overlook the importance of proper lens tint recommendations and low vision rehabilitaton to improve this patient’s quality of life.
Dr. Gerson is in private practice in Shawnee, Kan., and Kansas City, Mo. Dr. Shechtman is an associate professor at Nova Southeastern University College of Optometry in Ft. Lauderdale, Fla.
1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. 1993 Sep 30;329(14):977-86.
2. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998 Sep 12;352(9131):854-65.
3. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985 Dec;103(12):1796-806.
4. Chong EW, Kreis AJ, Wong TY, et al. Dietary omega-3 fatty acid and fish intake in primary prevention of age-related macular degeneration. A systemic review and meta-analysis. Arch Ophthalmol. 2008 Jun;126(6):826-33.
5. Schmidt-Erfurth U. Nutrition and retina. In: Augustin AJ (ed). Nutrition and the Eye—Basic and Clinical Research. Basel, Switzerland: Reinhardt Druck, 2005:120-47.
6. Thornton J, Edwards R, Mitchell P, et al. Smoking and age-related macular degeneration. A review of association. Eye. 2005 Sep;19(9):935-44.
7. Guymer RH, Chong EW. Modifiable risk factors for age-related macular degeneration. Med J Aust. 2006 May;184(9):455-8.
8. Francis PJ. The LOC387715 gene, smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Hered. 2007;63(3-4):212-8.
9. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36.
10. Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996 Nov 6;88(21):1550-9.
11. Richer S, Stiles W, Statkute L, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. 2004 Apr;75(4):216-30.
12. Stahl W. Macular Carotenoids: Lutein and Zeaxanthin. In: Augustin AJ (ed). Nutrition and the Eye—Basic and Clinical Research. Basel, Switzerland: Reinhardt Druck, 2005:70-88.
13. Berson EL. Vitamin A supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993 Nov;111(11):1456-9.
14. Berson EL, Rosner B, Sandberg MA, et al. Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment: subgroup analyses. Arch Ophthalmol. 2004 Sep;122(9):1306-14.

