 This month, we begin a bimonthly column on oculo-systemic disease, in which the authors demystify some of the complicated processes that lead to ocular involvement when systemic disease is present. Dr. Pelino is an assistant professor at the Eye Institute of Pennsylvania College of Optometry at
This month, we begin a bimonthly column on oculo-systemic disease, in which the authors demystify some of the complicated processes that lead to ocular involvement when systemic disease is present. Dr. Pelino is an assistant professor at the Eye Institute of Pennsylvania College of Optometry at
"Hal," a 70-year-old white male, presents with mild, gradually decreasing vision in both eyes of three years duration. His ocular history is positive for both cataract and age-related macular degeneration (AMD). His mother also became visually impaired due to AMD.
Twelve years earlier, Hal was diagnosed with hypertension, for which he takes Prinivil (lisinopril, Merck) 20mg qd. He takes no vitamins or supplements, has never smoked, and drinks one glass of red wine every night. Hal is due to see his internist in one week.
Best-corrected acuity is 20/25 O.U. Amsler grid testing is negative. Blood pressure is 147/82mm Hg. Hal is 6 feet tall and weighs 205 lbs. Both eyes show grade 1 cortical lens opacities and several small macular drusen.
After we obtained fundus photos, we prescribed ultraviolet protection and educated Hal about the ocular health benefits of improved blood pressure control, regular exercise, and a balanced diet that includes plenty of leafy green vegetables and fish. We also recommended a broad-spectrum antioxidant multivitamin that contains high levels of lutein and zeaxanthin, gave him a copy of the USDA food pyramid and information about age-related eye disease, and instructed him to return in six months. Finally, we dictated and faxed a letter to Hals internist, reporting both our findings and treatment plan.
A fairly typical scenario in your practice, right? Now, lets go back to 1986when the elder of us (Joe, in case you were wondering) saw his first AMD patient as an intern.
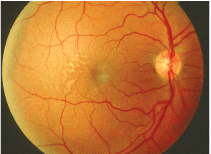
This patient with early AMD shows several small macular drusen. Is this a sign of more widespread metabolic disease?
At that point, we had a limited understanding of AMD. Few clinicians connected the pathogenesis with genetic, systemic health, nutritional, or lifestyle factors such as smoking. Though a handful of patients were stabilized with thermal laser photocoagulation, management consisted primarily of low-vision rehabilitation for advanced cases.
Now, just two decades later, we have a dramatically improved understanding of AMD. Research in the areas of molecular genetics, cardiovascular disease, immunology, nutrition, and the impact of a patients lifestyle has provided fresh perspectives in pathobiology. Significant strides have been made toward detecting AMD earlier, allowing for more timely intervention. Breakthrough pharmacotherapies have, for the first time, improved the visual function of AMD patients, enhancing their quality of life.
Paradigm Shift
Beginning in the late 1980s, there was a gradual paradigm shift in the way we think about AMD. Clinicians and researchers began to look beyond the retina for both causes and treatments. Since then, landmark studies have shown that the etiology and pathogenesis of AMD are a complex interaction of external and genetic factors.1,2
A growing body of evidence supports a link between AMD and cardiovascular disease. Oxidative stress and inflammation are likely the most common pathways.3 Indeed, inflammatory biomarkers associated with cardiovascular disease, namely C-reactive protein, homocysteine and interleukin-6, are also significantly elevated in patients with AMD.3 Molecular composition studies suggest that drusen are the result of local inflammation, and that the formation is a similar process as in other age-related diseases, such as atherosclerosis and Alzheimers disease, that also result in accumulation of extracellular plaques and deposits.4
Cigarette smoking in multiple large population studies has been consistently shown to be a risk factor for both AMD and cardiovascular disease.5
Recent research reveals a link between raised plasma levels of matrix metalloproteinase-9 (MMP-9) and AMD. MMPs are also implicated in the pathogenesis of atherosclerosis.6
What is good for the heart also appears to be good for the eye. Such positive measures include physical activity, antioxidants (including macular pigments zeaxanthin and lutein), omega-3 fatty acids (especially from fish), a reduced glycemic index diet, and good vascular compliance (healthy blood pressure, body mass index and cholesterol).7,8
In the Genes
In recent years, researchers have found that the complement factor H (CFH) complex, a group of genes related to the immune system, also is associated with AMD.9 This finding suggests that AMD is a systemic disease with a local manifestation at the central retina.10
So, why do mutations in complement genes selectively result in the phenotype of AMD? Perhaps the aging macula is particularly susceptible to damage because it exhibits decreased thickness and reduced integrity of the elastic layer of Bruchs membrane.10
Treatment Evolution
Only when clinicians and researchers began to view AMD as a systemic disease did significant advances occur in treatment. In the late 1980s, investigators determined that nonexudative (dry) AMD results from the gradual failure of the intracellular renewal function of the retinal pigment epithelium (RPE). Researchers hypothesized that harmful side effects of oxygen metabolism and solar radiation initiate free radical chain reactions that lead to degeneration of RPE cells.11
This type of early work led to the Age-Related Eye Disease Study (AREDS) and AREDS 2, as well as other studies that were designed to investigate whether antioxidants, which protect against free radicals, and other nutrients (i.e., lutein, zeaxanthin and omega-3 fatty acids) could lower the risk of AMD progression.12
Ocular-based treatments for the exudative (wet) form of AMD, such as thermal retinal laser, submacular surgery and photodynamic therapy, are now used less frequently in favor of delivering systemic medications into the vitreous. Anti-angiogenic drugs, such as corticosteroids and anti-VEGF (vascular endothelial growth factor) agents, have yielded improved visual outcomes.13
In the future, we can expect to see additional systemic-based treatments for AMD. Such preventative and therapeutic measures as angiotensin II type 1 receptor blockade, complement inhibition, and pharmacotherapies that target molecules involved in inflammation, are under investigation.14
Is AMD an ocular manifestation of a systemic disease? If so, this raises the possibility that it is part of a syndrome that affects other organs. What triggers the complement pathway in AMD? Is there a specific pathogen that causes the mechanism to activate? These answers may lead to new ways to prevent and treat AMD, and reduce vision loss.
1. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 2004 Apr; 122(4):598-614.
2. Seddon JM,
3.
4.
5. Thornton J, Edwards R, Mitchell P, et al. Smoking and age-related macular degeneration: a review of association. Eye 2005 Sep;19(9):935-44.
6. Chau KY, Sivaprasad S, Patel N, et al. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye 2008 Jun;22(6):855-9.
7. Tan JS, Wang JJ, Liew G, et al. Age-related macular degeneration and mortality from cardiovascular disease or stroke. Br J Ophthalmol 2008 Apr;92(4):509-12.
8. Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003 Dec;121(12):1728-37.
9. Jha P, Bora PS,
10. Scholl HP, Charbel Issa P, Walier M, et al. Systemic complement activation in age-related macular degeneration. PLoS ONE 2008 Jul 2;3(7):e2593.
11. Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol 1987 Mar-Apr;31(5):291-306.
12. Donaldson MJ, Pulido JS. Treatment of nonexudative (dry) age-related macular degeneration. Curr Opin Ophthalmol 2006 Jun;17(3):267-74.
13. Oliver SCN. Current diagnostic, preventive, and investigational therapies for age-related macular degeneration. In: Flynn HW (ed.). The Ophth Report 2008 Winter;1(2):12-18.
14. Kiss S. S Kiss. Emerging therapies for the treatment of age-related macular degeneration. The Ophth Report 2008 Winter;1(2):30-8.

