 A 39-year-old Middle Eastern male presented to the eye clinic for an updated refraction. He reported decreased vision while wearing his glasses or contact lenses (O.D. > O.S.), which he attributed to a change in refractive error.
A 39-year-old Middle Eastern male presented to the eye clinic for an updated refraction. He reported decreased vision while wearing his glasses or contact lenses (O.D. > O.S.), which he attributed to a change in refractive error.
He was in excellent health and took no medications. His ocular history was significant only for myopia, which––until now––was successfully corrected with glasses and contact lenses.
On examination, his best-corrected visual acuity measured 20/30 O.D. and 20/20 O.S. Extraocular motility testing was normal. Confrontation visual fields were full to careful finger counting O.U. His pupils were equally round and strongly reactive, with no afferent defect. The anterior segment examination was unremarkable.
Dilated fundus exam of the right eye showed small cups with good rim coloration and perfusion. However, we noted clinically significant changes (figure 1). Fundoscopic examination of the left eye was completely normal. Additionally, we performed a spectral-domain optical coherence tomography (SD-OCT) scan (figure 2) and a standardized echographic ultrasound (figures 3 and 4).
Take the Retina Quiz
1. What does the SD-OCT image of the right macula show?
a. Cystoid macular edema (CME).
b. Neurosensory retinal detachment.
c. Retinal pigment epithelium (RPE) detachment.
d. Choroidal mass pushing up the macula.
2. Based on the ultrasound, what is the lesion’s reflectivity?
a. Low.
b. Low to medium.
c. Medium.
d. High.
3. What is the most likely diagnosis for this patient?
a. Choroidal nevus.
b. Choroidal melanoma.
c. Choroidal hemangioma.
d. Choroidal metastasis.
4. How should this patient be managed?
a. Observation.
b. Enucleation.
c. Plaque radiotherapy.
d. Avastin (bevacizumab, Genentech/Roche) injection.
5. What is the overall prognosis for this patient?
a. Very poor.
b. Moderate.
c. Excellent.
d. Unknown.
For answers, see below.
Discussion
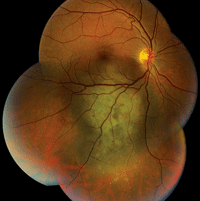
1. A fundus photograph of our patient’s right eye. Note the lesion located inferior to the macula, as well as the changes observed directly in the macula.
Clearly, our patient’s problem was more significant than an underpowered refractive correction. Upon examination, we detected an elevated pigment mass located along the inferotemporal arcade that extended peripherally. Additionally, the SD-OCT scan indicated the presence of a neurosensory retinal detachment that involved his macula. Judging by the clinical evidence, the lesion appeared to be a choroidal melanoma. On ultrasound testing, the lesion measured 16mm x 12.5mm x 4.1mm. It exhibited low to medium internal reflectivity, consistent with a choroidal melanoma.
With a definitive diagnosis in mind, a bigger question arose: How should we manage our patient? Do we recommend enucleation or plaque radiotherapy, and would either influence his chance of survival?
Enucleation seemed to be the obvious choice, with the idea that, “If it’s cancer, why wouldn’t you want it removed?” For this very reason, enucleation has been the preferred uveal melanoma treatment for more than 100 years. But what about the potential use of other “globe-preserving” modalities, such as plaque radiotherapy?
A little more than a decade ago, researchers from the National Eye Institute compared the therapeutic effects of enucleation and radioactive plaque therapy in the Collaborative Ocular Melanoma Study (COMS).
In one arm of COMS, 1,317 patients with medium-sized choroidal melanomas were randomized to receive either enucleation (660 patients) or iodine-125 plaque therapy (657 patients). At five-year follow-up, the survival rate of patients in both groups was equal, at approximately 90%––which is far better than what previous studies of ocular melanoma patients had shown.1 Given these impressive results, it seems that most patients would opt for iodine-125 plaque therapy vs. outright globe removal. Needless to say, our patient elected iodine-125 plaque therapy within the same week of his examination.
Our patient’s procedure was successful. So, he was in the clear, right? Unfortunately, a subsequent report from COMS indicated that just 45% of all uveal melanoma patients were alive and cancer-free after 12 years of treatment.2 As bleak as that statistic sounds, remember that just because a patient has died, it doesn’t necessarily mean that he or she passed away because of complications from metastatic disease. Indeed, many patients in COMS were elderly individuals who likely died of unrelated causes during the 12-year follow-up period.

2. The SD-OCT scan of the right macula revealed unique changes.
At 39 years of age, our patient probably has a long life ahead. Like all cancer patients, he always will be at risk for metastasis. But, is there a way to better predict if he will continue to live cancer-free? Thanks to several recent advances in molecular genetics: Yes.
Researchers have uncovered two distinct classes of uveal melanoma based on the tumor’s molecular genetics.3,4 Tumors in Class 1 carry a low risk of metastasis––less than 10%. Class 2 tumors, on the other hand, have a 90% likelihood of spreading to the liver.3,4
Molecular genetic testing has shown that aberrations on chromosomes 3, 6 and 8 are common in patients with uveal melanoma.4 In fact, gene expression profiling is now believed to be a more reliable predictive metric for uveal melanoma metastasis than any other clinical or histologic testing method.
Typically, molecular genetic testing is conducted using a fine-needle aspiration biopsy at the time of initial treatment. In this diagnostic procedure, a surgeon performs the biopsy with a 25-gauge needle that is passed directly into the tumor via a trans-scleral route.
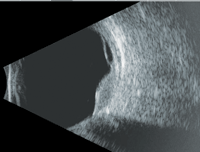
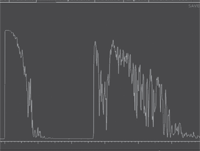
3, 4. Here’s a look at our patient’s ultrasound (B-scan left, A-scan right). What do you notice?
We performed molecular genetic testing on our patient at the time of his iodine-125 plaque therapy. Fortunately, the results suggested that he had a Class 1A molecular genetic profile––which exhibits just a 2% tumor-related mortality rate within five years of diagnosis.4 An ocular oncologist and a cancer specialist will continue to follow the patient closely for any signs of metastasis.
1. Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine-125 brachytherapy for choroidal melanoma, II: characteristics of patients enrolled and not enrolled. COMS Report No. 17. Arch Ophthalmol. 2001 Jul;119(7):951-65.
2. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006 Dec;124(12):1684-93.
3. Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004 Oct 15;64(20):7205-9.
4. Gill HS, Char DH. Uveal melanoma prognostication: from lesion size and cell type to molecular class. Can J Ophthalmol. 2012 Jun;47(3):246-53.
Answers
1. b
2. b
3. b
4. c
5. c

