Implantation of an investigational microstent significantly reduces intraocular pressure in patients with open-angle glaucoma, according to research presented at this year’s annual meeting of the American Academy of Ophthalmology in Chicago.
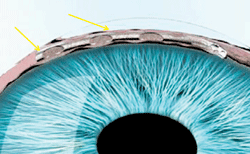
This stent, implanted into Schlemm’s canal, eliminated the need for topical drops in more than 70% of glaucoma patients.
Preliminary clinical trial results of the Hydrus Microstent (Ivantis) indicated that the device altogether eliminated the need for topical glaucoma medications in the majority of participants.
The microstent, described as an “intracanalicular scaffold” by the company, is inserted into Schlemm’s canal to increase outflow and reduce high IOP. It can be inserted using the same incisions as for cataract surgery. Currently, investigators are recruiting subjects for an ongoing Phase III clinical trial.1
In the recent study, the microstent was implanted in 69 patients with mild to moderate open-angle glaucoma. In 40 subjects, the device was implanted during cataract surgery, while the remaining 29 subjects had stent implantation without additional surgical intervention.
At six-month follow-up, patients who received both cataract surgery and stent implantation experienced a mean IOP reduction of 5.5mm Hg, from 21.1mm Hg to 15.6mm Hg. Those who had stent implantation alone experienced a mean IOP reduction of 4.7mm Hg, from 21.6mm Hg to 16.9mm Hg. No significant complications were reported.
Most impressively, 85% of combined surgery patients and 70% of stent-only patients no longer required topical glaucoma medications to control IOP levels. Further, they noted that the documented IOP reductions were sustained and consistent among all patients at one-year follow-up.
“So far, mini-stents appear to have important advantages in that they allow us to treat open-angle glaucoma at earlier stages and with lower complication risk,” said Thomas W. Samuelson, M.D., a founding partner of Minnesota Eye Consultants and medical monitor of the Hydrus clinical trial. “If the devices can effectively control IOP over many years, it would be a real breakthrough in combating this blinding disease.”
Crandall A. Safety and effectiveness study of the Hydrus device for lowering IOP in glaucoma patients undergoing cataract surgery. Clinical Trials identifier: NCT01539239. Available at:
http://clinicaltrials.gov/ct2/show/NCT01539239. Accessed November 30, 2012.

