Glaucoma management has been rapidly changing in recent years. An increase in surgical glaucoma treatment options has made it difficult to navigate the constantly changing terrain of glaucoma management. A 2017 survey of glaucoma surgeons showed that minimally invasive glaucoma surgeries (MIGS) are on the rise, with 22% of cataract surgeries performed by glaucoma specialists including a MIGS procedure.1 But with more than 10 different MIGS on the market in the United States, how do you know which one would benefit your patient? This article discusses the recent studies highlighting the benefits and risks of several MIGS procedures to help you better counsel patients on their options.
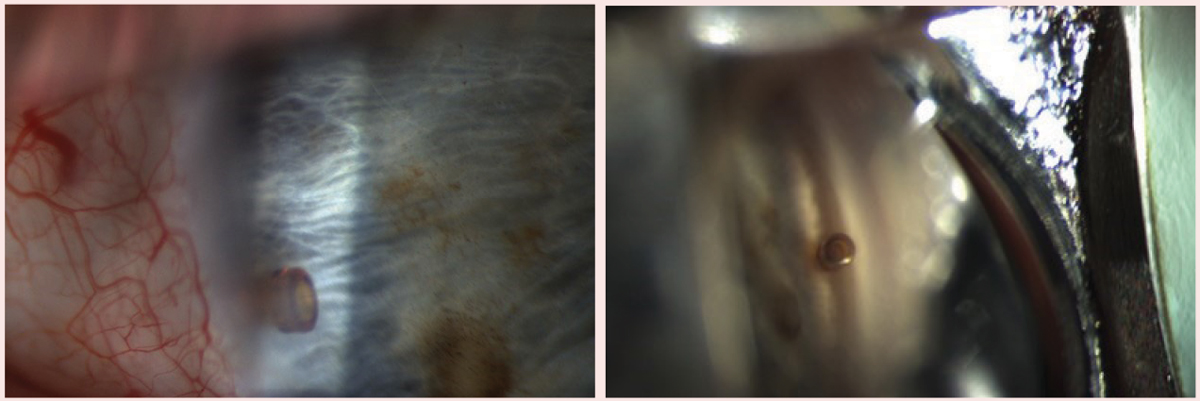
 |
| iStent inject insertion directly following cataract surgery. Click image to enlarge. |
Trabecular Meshwork Bypass
Several procedures seek to increase aqueous outflow with this method:
iStent (Glaukos). This device remains a good treatment option for patients with mild-to-moderate glaucoma who use one to three medications and require cataract surgery. As the first FDA-approved trabecular meshwork (TM) bypass MIGS device, many surgeons are comfortable with it, which is why it is frequently used today. Several studies show greater intraocular pressure (IOP) and medication reduction in patients with primary open-angle glaucoma (POAG) who receive the iStent combined with cataract surgery than in glaucoma patients who had cataract surgery alone.2
One recent study shows the original iStent is slightly more effective than the iStent inject (Glaukos) in lowering IOP; however, the results were not statistically significant.3 This study also shows intraoperative bleeding and postoperative hyphema are about twice as common in the iStent group compared with the iStent inject group, but these complications were not significant and self-resolved without sequelae.
iStent inject. This second-generation TM bypass device is similar in appearance to a punctal plug. The surgeon inserts the device into the nasal angle of the TM during cataract surgery. Due to the ease of insertion, the iStent inject is gaining popularity among surgeons. One study shows favorable two-year IOP reduction outcomes with an iStent inject combined with cataract surgery vs. cataract surgery alone. Unmedicated IOP two years post-op was reduced by 7.0±4.0mm Hg in those with the combined treatment vs. 5.4±3.7mm Hg in those with cataract surgery alone.4 Complications are rare, but the most common include malposition of the stent as well as with blood reflux during and after the procedure.5
One study shows the iStent inject combined with cataract surgery to be effective in the treatment of primary and secondary glaucoma, including POAG, ocular hypertension, angle closure glaucoma, pseudoexfolative, normal tension and combined mechanism glaucoma. This study included patients with mild, moderate and severe glaucoma. IOP decreased from 19.95±3.7mm Hg to 16.75±2.2mm Hg and medications were reduced from 1.3±0.66mm Hg to 0.3±0.57mm Hg. All patients had at least 20% IOP reduction from baseline. The study reported no adverse events.6
MIGS: iStent Leads the PackMinimally invasive surgeries have gained momentum in recent years, offering a new approach for some glaucoma patients. But with so many differing procedures and devices, it can be hard to keep up with usage patterns among surgeons. The American Glaucoma Society (AGS)–IRIS Registry study may help, as it provides insight into the demographic differences in three areas: MIGS procedure usage, effectiveness and adverse outcomes/safety concerns. In total, 383,942 patients (591,116 eyes; 88.69%) received cataract surgery alone, while 50,970 patients (75,358 eyes; 11.31%) underwent combined cataract and MIGS procedures. Most (51,897 eyes) received the iStent, followed by endoscopic cyclophotocoagulation (BVI Medical) (15,714 eyes), according to Mildred M.G. Olivier, MD, who presented the findings during the 2019 American Academy of Ophthalmology annual meeting. The data also shows the highest rates of MIGS usage tend to occur in patients who are male, black, older than age 60 and from the Midwest. Additionally, they typically had mild to moderate disease, were covered by Medicare and seen by a non-glaucoma specialist, Dr. Olivier noted. The findings highlight the growing popularity of this approach, with an increase in MIGS use from 5.2% to 14.9% during the study period |
Hydrus (Ivantis). This 8mm bypass device also serves as a three clock-hour Schlemm’s canal scaffold. A prospective multicenter randomized trial compared patients who underwent Hydrus implantation and cataract surgery vs. cataract surgery alone. Two years after surgery, IOP was reduced by 7.6±4.1mm Hg in the Hydrus group compared with 5.3±3.9mm Hg in the group with cataract surgery alone.7 There were 1.4 fewer medications in the combined procedure group compared with 1.0 fewer medications in the cataract surgery-only group.
The most common intraoperative adverse events observed in the study were device malposition (1.6%) and hyphema (1.1%), but there were no reported long-term sequelae from these complications. Nonobstructive peripheral anterior synechiae (PAS) was the most common post-operative complication (14.9%). A concern among surgeons is the difficulty of insertion of the device due to its length; however, one study shows that the learning curve of insertion by an experienced glaucoma surgeon is negligible.8 The results of this study show promise of Hydrus’s use increasing among general cataract surgeons.
Complications. The most common postoperative complications of iStent, iStent inject and Hydrus are hyphema, stent malposition or obstruction and IOP elevation. Hyphema after surgery is most often transient and self-resolving. If recurrent or severe hyphema occurs, the device may require removal. Stent obstruction is an infrequent complication but can lead to device failure. Early obstruction is typically due to device malposition; whereas, chronic inflammation can lead to PAS and later obstruction. If the obstruction is observable, the lumen may be treated with a YAG laser. Few studies have reported severe but transient elevated IOP after iStent insertion.9 Observing elevated IOP one day after surgery may warrant anterior segment decompression to lower IOP.
 |
| Hydrus insertion directly following cataract extraction.Click image to enlarge. |
Trabeculotomy
These procedures target the trabecular meshwork/Schlemm’s canal:
Trabectome (Microsurgical Technology). The FDA approved this MIGS in 2004 for partial trabeculotomy in patients with mild to moderate glaucoma with or without cataract surgery. The Trabectome device cauterizes and aspirates the TM tissue anywhere from 90 to 180 degrees on the nasal angle. One study shows Trabectome is effective in uveitic glaucoma patients where other trabeculotomy procedures may not be as effective. Removal of excess tissue reduces the risk of PAS formation in the angle.10 Another study shows sustained long-term IOP reduction from 20.0±5.6mm Hg pre-op to 15.6±4.6mm Hg post-op and medication reduction from 1.8±1.2 to 1.0±1.2 at five years post-op.11
Reported potential complications of Trabectome are blood reflux into the anterior chamber, hyphema and partial goniosynechiae. None of these were clinically significant in the treatment of 101 patients with POAG.12 Some surgeons avoid Trabectome due to the thermal damage to the angle compared with other trabeculotomy procedures. A new probe is under development to reduce this damage. Trabectome was the first FDA-approved MIGS and may not be as popular in some areas due to the development of newer MIGS procedures and stents.
Kahook Dual Blade (New World Medical). This procedure has two advantages: (1) reduced cost compared with other procedures and (2) full removal of the TM tissue, up to 180 degrees, without thermal damage. Kahook can be performed alone or during cataract extraction. One recent study shows favorable results of Kahook as a stand-alone procedure for POAG, where the mean IOP was reduced from 23.5±1.1mm Hg to 15.0±0.6mm Hg six months after the procedure.13 Hyphema is an expected surgical complication and typically self-resolves within a few days to weeks.
Goniotome (Microsurgical Technology). This device is like the Trabectome with aspiration and irrigation ports but excises the TM in the same fashion as the Kahook. This allows for better surgical views and homeostasis during the procedure and aids in removal of excised trabecular tissue. The IOP-lowering effect of trabeculotomy performed with Goniotome would mirror those of Kahook.
Gonioscopy-assisted transluminal trabeculotomy (GATT). For many years, this was considered a treatment for congenital glaucoma, but it is now an effective treatment for POAG in adults. This procedure consists of inserting a suture or catheter through Schlemm’s canal and extracting the suture (or device), which then unroofs the entire TM 360 degrees. One recent study shows significant IOP reduction two years after GATT by an average of 9.2mm Hg in patients with POAG and medications were reduced an average of 1.43. The same study shows secondary glaucoma patients’ IOP reduced by 14.1mm Hg and two fewer medications per patient on average.14
The main advantage of this procedure is that it treats 360 degrees of the angle, where other methods of trabeculotomy only treat up to 180 degrees. Because GATT can be performed without specialty equipment, it may be the most cost-effective MIGS and a key factor why its use has increased among surgeons. Potential disadvantages include remnants of the TM that may adhere to the adjacent tissues and cause PAS. In studies where this complication was observed, the effects were not clinically significant. One study showed that up to 16% of patients do not have patent Schlemm’s canal 360 degrees. Limited Schlemm’s canal patency will limit the treatable area of TM with GATT, potentially reducing the effectiveness of the procedure in these patients.15
An alternative to performing GATT alone is by combining Trabectome with GATT to treat the angle, also known as Trabectome-initiated GATT. The surgeon performs a 90-degree trabeculotomy with Trabectome and then treats the residual 270 degrees of the TM with GATT. This procedure was developed to ensure at least 90 degrees of the angle would be treated if Schlemm’s canal is not open to perform a complete GATT.16
Ab interno canaloplasty (ABiC, iTrack). This is a modification of traditional canaloplasty, as it achieves the same viscodilation of Schlemm’s canal with an internal approach. Studies shows similar outcomes with and without the suture in traditional canaloplasty.17 ABiC is the only MIGS procedure that treats all structures of the aqueous outflow system. During the procedure, adhesions in the TM are broken, the Schlemm’s canal is dilated and collector channels are irrigated. Unlike many of the MIGS procedures, ABiC can be repeated.
Few studies evaluating the results of ABiC have been published, but one recent study shows IOP reduction from 18.5±3.4mm Hg to 13.8±2.0mm Hg after surgery. Medications were also reduced from 2.4 to 0.25 on average.18 Another ABiC study shows similar outcomes in POAG patients treated with ABiC in one eye and traditional canaloplasty in the other.19 Complications associated with ABiC are rare, and further long-term studies are needed to determine any associated significant complications.
Omni (Sight Sciences). This device is designed to viscodilate Schlemm’s canal and collector channels and perform a trabeculotomy simultaneously. With this device, the surgeon can choose to treat 180 or 360 degrees of the angle with Visco 360 and/or Trab 360 (Sight Sciences).
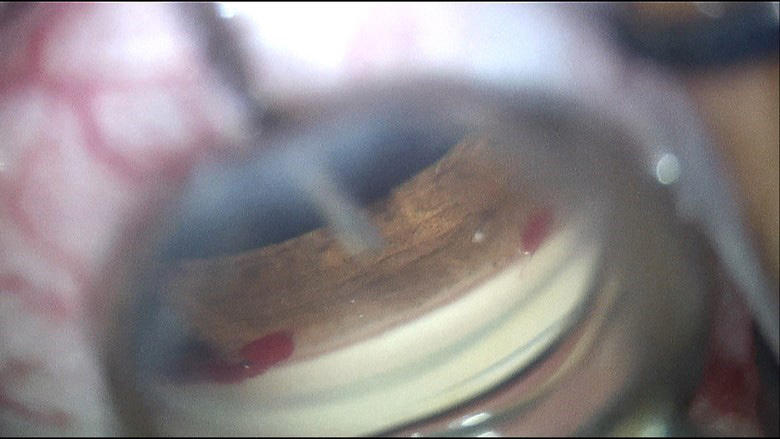
 |
| Ab interno canaloplasty directly following cataract surgery. Click image to enlarge. |
Cyclophotocoagulation
Endocyclophotocoagulation (ECP, BVI Medical) reduces aqueous outflow and is the only MIGS with indications in neovascular and angle-closure glaucoma. Because ECP directly treats the ciliary processes, some surgeons feel it is less traumatic than transscleral cyclophotocoagulation (CPC). ECP can be performed with or without cataract extraction; however, it is better suited for pseudophakic eyes or during cataract extraction because the energy applied to the eye may induce cataract development.
Research shows combining phaco and ECP can open the angle in the plateau iris by shrinking the ciliary processes.20 This combination can be more effective in chronic angle-closure patients vs. POAG patients in both lowering IOP and medications.21 Due to possible complications such as inflammation, cystoid macula edema, cataract development, hypotony and phthisis bulbi, practitioners are advised to perform ECP with the least amount of energy to achieve effective treatment.
CPC uses diode laser energy applied to the external sclera approximately 1.5mm posterior to the limbus with the Cyclo G6 Glaucoma Laser System (Iridex). One study showed CPC to have three modes of action: reducing aqueous production, increasing uveoscleral outflow and causing a pilocarpine-type effect. Although not considered a MIGS procedure by some (CPC is performed externally), it can be an effective means of lowering IOP by reducing aqueous outflow. CPC can also effectively deal with refractory glaucoma and narrow-angle glaucoma.22 Potential complications are hypotony, phthisis bulbi and chronic inflammation, all of which can be reduced by titrating the amount of energy exposed to the eye. However, more studies are needed on this procedure with published guidelines for treatment titration, as all reported complications are dose-dependent.23
Subconjunctiva
The Xen gel stent (Allergan) is a hydrophilic 6mm tube placed ab-internally to create a subconjunctival bleb. Many surgeons consider Xen to be a MIGS plus procedure due to the bleb formation, which comes with increased risk of complications compared with other MIGS. Xen is FDA approved for insertion in patients with refractory glaucoma as an independent procedure or during cataract surgery. One recent study shows a significant reduction in IOP from 22.5±4.2mm Hg to 13.4±1.3mm Hg four years postoperatively. Medication use also decreased in the treatment group from an average of 2.4±1.3 before surgery to 1.2±1.3 after surgery.24
Another study shows that 43% of Xen gel stent blebs required needling postoperatively to break fibrotic adhesions and aid in reforming the bleb.25 Many general surgeons may not feel comfortable with bleb needling, so a glaucoma specialist usually performs this procedure.
Reported complications with the Xen gel stent are malignant glaucoma, conjunctival wound leak, hyphema, vitreous hemorrhage, hypotony maculopathy, choroidal effusion stent obstruction, exposed stent and dellen formation.26 In light of the potential risks associated with the complications of the Xen gel stent, these are best managed by the surgeon who implanted the stent. In October 2019, Allergan announced a voluntary recall of 15 lots of Xen implants due to residual polishing materials on the needle sleeve from the manufacturing process. The recall was for unused stents, and explanting devices is not advised at this time.27
Suprachoroidal StentsCurrently, suprachoroidal MIGS devices are not available in the United States. CyPass (Alcon), a suprachoroidal stent, was voluntarily removed from the market in August 2018 due to increased endothelial cell loss noted in some patients five years after implantation.
The American Society of Cataract and Refractive Surgeons task force recommended clinicians monitor eyes that have a CyPass stent to watch for visually significant complications from endothelial cell loss. Repositioning or removing the device is discouraged; instead, the surgeon may attempt to clip the proximal end of the device, reducing any protrusion into the anterior chamber.
|
Future of MIGS
Many other devices are still under investigation:
- Standalone procedures for iStent and iStent inject are in clinical trials.
- The iStent infinite (Glaukos), a device housing three stents similar to the iStent inject, is currently going through FDA trials for use in refractory glaucoma. When approved, this device will allow for iStent implantation as a standalone procedure.
- The iStent supra is a suprachoroidal device currently in the final stages of the FDA approval process. Glaukos anticipates its release sometime in 2020. This device will fill the void CyPass left; however, some surgeons may be leery of potential complications caused by suprachoroidal stents.
- The MicroShunt (Santen, Glaukos) is a an ab externo subconjunctival stent that is currently seeking FDA approval. After approval, this device will be a good alternative to Xen gel stent.
- The SolX Gold Shunt (SolX) is a suprachoroidal device in clinical trials for FDA approval.
- iDose (Glaukos) is a medication implant device in clinical trials for long-term drug administration in vivo into the anterior chamber. The time benefit for iDose will be limited when the drug release is exhausted, but it may be used in cases where medication compliance is an issue.
- The Beacon Aqueous Microshunt (MicroOptx) is a novel device that filters aqueous from the anterior chamber to the surface of the eye. The device is implanted in the cornea stroma, is designed to maintain the IOP at 12mm Hg and shows great promise for a new MIGS device.
Glaucoma surgeons’ proficiency varies greatly on the procedures they perform, from no MIGS to several. Surgeons usually become proficient in a few procedures rather than trying to master them all.28 Comanaging optometrists should know which MIGS procedures are available before referring patients.
Glaucoma treatment options are ever changing, and optometrists must be knowledgeable on all of the MIGS on the market to provide the best possible care to each patient.
Dr. Caywood is a staff optometrist at the Oklahoma City VA Medical Center.
Surgery videos performed by Andrew Bailey, MD, a glaucoma specialist at Dean McGee Eye Institute in Oklahoma City, OK.
| 1. Vinod K, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society. J Glaucoma. 2017;26(8):687-93. 2. Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. 3. Hooshmand J, Rothschild P, Allen P, et al. Minimally invasive glaucoma surgery: comparison of iStent with iStent inject in primary open angle glaucoma. Clin Exp Ophthalmol. 2019;47(7):898-903. 4. Samuelson TW, Sarkisian SR Jr, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811-21. 5. Skinner A. How to manage MIGS complications. Rev Ophthalmol. 2019;25(3):50-7. 6. Clement CI, Howes F, Ioannidis AS, et al. One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multi-surgeon study. Clin Ophthalmol. 2019;13:491-9. 7. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON Study. Ophthalmology. 2019;126(1):29-37. 8. Al-Mugheiry TS, Cate H, Clark A, Broadway DC. Microinvasive glaucoma stent (MIGS) surgery with concomitant phakoemulsification cataract extraction: outcomes and the learning curve. J Glaucoma. 2017;26(7):646-51. 9. Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339-45. 10. Anton A, Heinzelmann S, Neß T, et al. Trabeculectomy ab interno with the Trabectome as a therapeutic option for uveitic secondary glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1973-8. 11. Esfandiari H, Shah P, Torkian P, et al. Five-year clinical outcomes of combined phacoemulsification and Trabectome surgery at a single glaucoma center. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):357-62. 12. Minckler D, Baerveldt G, Ramirez MA, et al. Clinical results with the Trabectome, a novel surgical device for treatment of open-angle glaucoma. Trans Am Ophthalmol Soc. 2006;104:40-50. 13. Berdahl JP, Gallardo MJ, ElMallah MK, et al. Six-month outcomes of goniotomy performed with the Kahook Dual Blade as a stand-alone glaucoma procedure. Adv Ther. 2018;35(11):2093-102. 14. Grover DS, Smith O, Fellman RL, et al. Gonioscopy-assisted transluminal trabeculotomy: an ab Interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma. 2018;27(5):393-401. 15. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: interim clinical study analysis. J Cataract Refract Surg. 2007;33(7):1217-26. 16. Smith BL, Ellyson AC, Kim WI. Trabectome-initiated gonioscopy-assisted transluminal trabeculotomy. Mil Med. 2018;183(suppl_1):146-9. 17. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: three-year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg. 2011;37(4):682-90. 18. Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd. 2018;32(6):223-7. 19. Gallardo MJ, Supnet RA, Ahmed IIK. Circumferential viscodilation of Schlemm’s canal for open-angle glaucoma: ab-interno vs. ab-externo canaloplasty with tensioning suture. Clin Ophthalmol. 2018;12:2493-8. 20. Francis BA, Pouw A, Jenkins D, et al. Endoscopic cycloplasty (ECPL) and lens extraction in the treatment of severe plateau iris syndrome. J Glaucoma. 2016;25(3):e128-33. 21. Lin MM, Rageh A, Turalba AV, et al. Differential efficacy of combined phacoemulsification and endocyclophotocoagulation in open-angle glaucoma versus angle-closure glaucoma. J Glaucoma. 2019;28(5):473-80. 22. Bezci Aygün F, Mocan MC, Kocabeyoglu S, Irkeç M. Efficacy of 180° cyclodiode transscleral photocoagulation for refractory glaucoma. Turk J Ophthalmol. 2018;48(6):299-303. 23. Sanchez FG, Grippo TM. Micropulse transscleral CPC: an evidence review. Glaucoma Today. 2019;17(2):34-8. 24. Lenzhofer M, Kersten-Gomez I, Sheybani A, et al. Four-year results of a minimally invasive transscleral glaucoma gel stent implantation in a prospective multi-centre study. Clin Exp Ophthalmol. 2019;47(5):581-7. 25. Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579-88. 26. Yook E, Vinod K, Panarelli JF. Complications of micro-invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29(2):147-54. 27. Opthalmic Mutual Insurance Company. Xen glaucoma device recall. www.omic.com/xen-glaucoma-device-recall. Accessed November 18, 2019. 28. Kent C. Choosing MIGS (for you and your patient). Rev Ophthalmol. 2018;24(6):20-33. |