 |
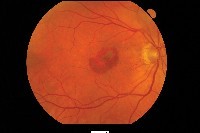
| Choroidal neovascularization affects 10% of those who have AMD but causes 90% of severe vision loss. |
Before April 2000, laser photocoagulation was the only approved treatment for CNV (although surgeons also performed submacular surgery, macular translocation and transpupillary thermotherapy). However, only 13% of patients with CNV would qualify for photocoagulation when applying guidelines from the Macular Photocoagulation Study (MPS), which recommended treatment only for fully classic CNV.2,3 This type of CNV, which is completely visible in the early phase of fluorescein angiography (FA), grows more aggressively than other types of CNV but occurs less commonly. Also, laser photocoagulation ablates choroidal and retinal tissue along with CNV, causing patients who have CNV underneath the fovea to lose an average of three lines of vision immediately after treatment.
The introduction of photodynamic therapy (PDT) and drug treatments that inhibit CNV now offer patients new hope. Here, well look at options that are available to our patients as well as on the horizon.
Visudyne PDT
PDT with Visudyne (verteporfin, Novartis Ophthalmics), which received FDA approval in April 2000, became the first vision-sparing technique for subfoveal CNV. Visudyne was initially approved for treating subfoveal predominantly classic CNV (50% or more of the CNV is seen in the early phase of FA). However, later reports from three studiesTAP (Treatment of Age-Related Macular Degeneration with Photodynamic Therapy), VIP (Verteporfin in Photodynamic Therapy) and VIM (Visudyne in Minimally Classic Trial)provided evidence that in other types of CNV (<4.00DD), results were favorable regardless of how much classical component there was or in certain cases of occult CNV. This led to updated recommendations from the Verteporfin Roundtable Participants (a group brought together by Novartis).4
In Visudyne PDT, the surgeon injects the verteporfin dye into the bloodstream, where it binds to the low-density lipoprotein that selectively accumulates in neovascular tissue, then activates the dye with a low-intensity laser. The verteporfin reacts with oxygen, resulting in cell death and CNV thrombosis.
Initial reports showed a benefit for patients who had predominantly classic CNV only; 59% of patients treated with PDT every three months for two years lost less than three lines of vision vs. 31% of patients who received a placebo.5 Later reports showed a benefit for patients with less than 50% classic CNV provided the CNV covered a smaller area.6
Compared to thermal laser, PDT treats many more patients. One study of 168 eyes with CNV found that the MPS guidelines would have allowed five eyes (3%) to be treated with thermal laser, yet Visudyne practice guidelines from the American Academy of Ophthalmology would have qualified up to 77 eyes (43%).7
Despite its benefits, some 39% of patients in one study lost more than three lines of vision after two years of treatment with Visudyne.8 But, researchers continue to look at ways to improve the outcomes, including:
Reduced laser intensity. A recent study found that reducing the laser intensity by half provided better results in patients who had minimally classic CNV after two years, regardless of CNV size at the start of the study.8 Results of a study of patients with fully occult CNV treated with normal laser intensity are pending.9
Combined treatment with intravitreal Kenalog injections. This has become common practice. Kenalog (triamcinolone acetonide), a steroid, targets the intracellular process involved in the development of CNV. It likely inhibits platelet-derived and fibroblast-derived growth factors, downregulates matrix metalloprotease, and inhibits vascular endothelial growth factor (VEGF)-induced breakdown of the blood-ocular barrier.
Studies have shown that Kenalog may lead to the resolution of subretinal and intraretinal fluid, resulting in better visual outcomes, and the need for fewer PDT treatments.5,6,8,10-14 However, much of this data is not based on comparison to controls; rather, they are based just on what we know from previous studies on Visudyne PDT.
While Kenalog injections, like other steroids, can lead to cataract progression and IOP elevation, they have not been associated with a significant rate of endophthalmitis.15
Icon-targeted PDT (ITPDT). This method, which is in a pre-clinical trial phase, employs an Icon protein-verteporfin conjugate to destroy CNV tissue in a more selective method. The Icon protein binds to tissue factor, which is expressed on endothelial cells of CNV but not on the endothelial cells of normal vasculature. ITPDT successfully inhibited CNV growth for one week in rats.16
Another PDT Option
A second PDT treatment may soon be available to patients. The FDA issued an approvable letter for Photrex (rostaporfin, Miravant Medical Technologies) in September 2004, and final approval is pending.
Photrex (formerly SnET2) has a similar mechanism of action to Visudyne. In clinical trials, 58% of patients lost less than three lines of visual acuity after two years vs. 42% in the placebo group. The trials were not restricted to any specific type of CNV, and patients with classic CNV benefited regardless of how much classic component there was.17
Patients who had better baseline visual acuity benefited more from the treatment. They required an average of 2.8 treatments with Photrex over two years, significantly fewer than the number of Visudyne treatments necessary (according to the TAP and VIP studies, 4.9 and 5.6 treatments, respectively). But, Photrex has a longer half-life, so patients will experience photosensitivity for about 10 days vs. five days with Visudyne.
| Comparison of Two Photodynamic Therapies |
| Visudyne (verteporfin) | Photrex (rostaporfin) | |
| Wavelength used | 689nm | 664nm |
| Expected # of days to avoid sunlight | Five | 10 |
| Average # of treatments in studies | 4.9 to 5.6/two years | 2.8/two years |
| Predictors for better VA outcome | CNV < 4 disc area (DA) | Better baseline VA |
| Percent of patients who lost <3 lines of VA in two years (treatment vs. placebo) |
| For all types of CNV | 53% vs. 38% | 58% vs. 42% |
| For predominantly classic | 59% vs. 31% | Not reported yet |
| For minimally classic | 19% vs. 17%* | 64% vs. 29% |
| For fully occult | 45% vs. 31%* | 65% vs. 0% |
| * Difference not statistically significant. |
Macugen
As researchers gain a new understanding about the underlying process of CNV, new drug therapies are emerging to inhibit the process. The first such therapy available: Macugen (pegaptanib sodium, Eyetech/Pfizer), which received FDA approval in December 2004 for treating all types of CNV.
Macugen is an aptamer (a DNA or RNA molecule that binds to a protein). It binds to VEGF, the protein believed to be one of the main mediators for pathological intraocular vascularization, and blocks it from stimulating the receptor on the surface of the endothelial cell. It is administered as an intravitreal injection every six weeks.
The VEGF Inhibition Study in Ocular Neovascularization-1 (VISION-1) found that 70% of patients (all types of CNV) treated with Macugen for a year lost less than three lines of vision vs. 55% in the placebo group.18 A one-year follow-up study re-randomized patients to treatment or placebo and found a sustained benefit in patients who received treatment for two years.19 Only 1.3% of patients developed endophthalmitis, 0.6% developed retinal detachment, 0.6% developed traumatic cataract, and no patients developed elevated IOP, although these complications remain a concern with intravitreal injections.20
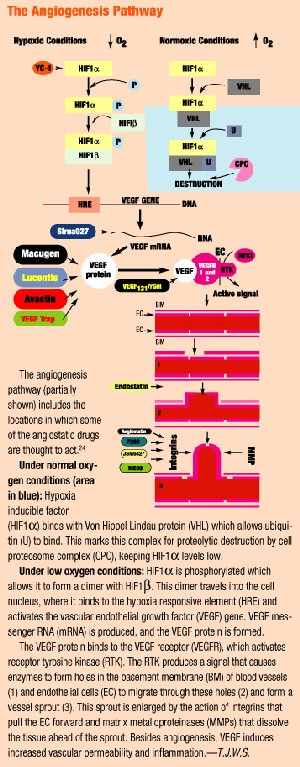 |
Lucentis
Lucentis (ranibizumab, Genentech/Novartis), also known as rhuFab V2, is an anti-VEGF antibody that is applied as an intravitreal injection every four weeks.
Preliminary results from a one-year Phase III clinical trial showed that 95% of patients who had minimally classic or occult CNV lost less than three lines of vision vs. 60% of control subjects. Also significant: On average, patients treated with Lucentis gained vision, while patients treated with a placebo lost vision.
Two additional Phase III clinical trials of Lucentis are under way: the ANCHOR Trial,which will compare two different doses of Lucentis to PDT, and the PIER Trial, which will evaluate patients with subfoveal CNV (with or without a classic component) who receive Lucentis once a month for the first three doses, then once every three months for two years. Results are expected in the last quarter of 2005 and the first half of 2006, respectively.
Retaane
The FDA granted conditional approval of Retaane 15mg (anecortave acetate, Alcon), an angiostatic corticosteroid used to inhibit CNV, on May 24, 2005. Retaane is administered as a periocular posterior juxtascleral depot injection every six months.
Earlier clinical trials showed that reflux of the drug was a problem, so Alcon developed a counter-pressure device (CPD). One randomized, 24-month study of patients with all types of CNV found that 90% of patients who received treatment lost less than three lines of vision vs. 53% of those who received a placebo. But, when the CPD was not used, only 74% of patients lost less than three lines of vision.
Researchers also performed a one-year, randomized study that compared Visudyne PDT, Retaane injections and sham treatments; they found that Visudyne and Retaane were comparable in efficacy.21 Specifically, 49% of patients who received Visudyne PDT, 50% of patients who received Retaane injections (with the CPD) and 39% of patients who received Retaane (without the CPD) lost less than three lines of acuity.
The Risk Reduction Trial, a four-year study initiated in 2004, will evaluate whether treatment with Retaane every six months reduces the risk for developing CNV in patients who have the dry form of AMD.22
Evizon
Evizon (squalamine, Genaera Corporation) is an anti-angiogenesis drug for AMD. Squalamine lactate is a synthetic molecule that inhibits several steps in the angiogenic process, including the VEGF signal pathway, integrin expression and cytoskeletal formation. Integrins are adhesion molecules that aid in the development of the neovascular bud during the formation of CNV. The result: inactivation of endothelial proliferation that leads to neovascularization. Squalamine does not cause regression of neovascular tissue that already is present. Unlike other treatments, Evizon is systemically (intravenously) rather than ocularly administered.
Interim results of a Phase II, non-placebo-controlled clinical trial revealed that 26% of patients with all types of CNV who received Evizon once a week for four weeks had three or more lines of improvement in visual acuity. The remaining 74% maintained their initial visual acuity or had less than a three-line loss at the four-month visit. No patient lost more than three lines of vision.23
In comparison, after four months of treatment with Visudyne, between 13% (predominantly classic CNV) and 18% (minimally classic CNV) of treated patients already had lost more than three lines of vision.5,6,8
Two additional Phase II clinical trials of Evizon are under way in the United States. A two-year study will evaluate the effectiveness of two doses of Evizon a week for four weeks, followed by one dose every four weeks until week 48. Another 18-month trial will evaluate the use of Evizon with concomitant Visudyne PDT treatment followed by monthly Evizon doses for six months.
|
Additional AMD Treatments |
Options for AMD patients were once limited, but these new treatments hold great promise for improving visual outcomes and possibly preventing CNV. We must stay current with this rapidly progressing field to offer our patients the most appropriate options.
Dr. Stokkermans is a clinical assistant professor of ophthalmology at Case School of Medicine and a staff optometrist at University Hospitals, both of which are in Cleveland.
1. Congdon N, OColmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004 Apr;122(4):477-85.
2. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol 1993 Sep;111(9):1200-9.
3. Freund KB, Yannuzzi LA, Sorenson JA. Age-related macular degeneration and choroidal neovascularization. Am J Ophthalmol 1993 Jun 15;115(6):786-91.
4. Verteporfin Roundtable Participants. Guidelines for using verteporfin (Visudyne) in photodynamic therapy for choroidal neovascularization due to age-related macular degeneration and other causes: update. Retina 2005 Feb-Mar;25(2):119-34.
5. Bressler NM; Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol 2001 Feb;119(2):198-207.
6. Blinder KJ, Bradley S, Bressler NM, et al. Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP report no. 1. Am J Ophthalmol. 2003 Sep;136(3):407-18.
7. Zawinka C, Ergun E, Stur M. Prevalence of patients presenting with neovascular age-related macular degeneration in an urban population. Retina 2005 Apr-May;25(3):324-31.
8. Azab M, Boyer DS, Bressler NM, et al. Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial. Arch Ophthalmol 2005 Apr;123(4):448-57.
9. Kaiser PK, VIO Study Group. Verteporfin In Occult (VIO): the design of a phase III controlled clinical trial of verteporfin therapy for occult with no classic subfoveal CNV secondary to AMD. ARVO Abstract #2276, 2004.
10. Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology 2005 Feb;112(2):301-4.
11. Rechtman E, Danis RP, Pratt LM, Harris A. Intravitreal triamcinolone with photodynamic therapy for subfoveal choroidal neovascularisation in age related macular degeneration. Br J Ophthalmol 2004 Mar;88(3):344-7.
12.Chaudhary V, Mao A, Hooper PL, Sheidow T. The effect of triamcinolone acetonide as an adjunctive treatment to verteporfin therapy in neovascular age-related macular degeneration: a prospective, randomized, placebo controlled pilot clinical trial. ARVO Abstract #2308, 2005.
13. Ufret RL, Williams D, Kaiser PK. OCT and angiographic analysis of neovascular AMD treated with PDT in combination with intravitreal triamcinolone acetonide vs. PDT alone. ARVO Abstract #316, 2005.
14. Moshfeghi AA, Puliafito CA, Rosenfeld PJ. Verteporfin therapy with intravitreal triamcinolone in patients with neovascular AMD. ARVO Abstract #3159, 2004.
15. Westfall AC. Acute endophthalmitis incidence after intravitreal triamcinolone acetonide. ARVO Abstract #5562, 2005.
16. Lu F, Hu Z, Garen A, Adelman RA. Icon-targeted photodynamic therapy for treatment of choroidal neovascularization in a laser-induced rat model. ARVO Abstract #3026, 2005.
17. Thomas EL, Danis RP and SnET2 Study Group. Lesion progression and visual acuity outcomes for occult choroidal neovascularization treated with rostaporfin (SnET2) photodynamic therapy. ARVO Abstract #3571, 205.
18. Gragoudas ES. VEGF Inhibition Study in Ocular Neovascularization-1 (VISION1): efficacy results from phase II/III Macugen (pegaptanib sodium) clinical trials. ARVO Abstract #2364, 2004.
19. DAmico DJ, VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group. VEGF Inhibition Study in Ocular Neovascularization (VISION): second year efficacy data. ARVO Abstract #2309, 2005.
20. DAmico DJ, Bird AC. VEGF Inhibition Study in Ocular Neovascularization1 (VISION1): safety evaluation from the pivotal Macugen (pegaptanib sodium) clinical trials. ARVO Abstract #2363, 2004.
21. Bochow TW, Harper CA III, Marks BB, et al. Reflux of study medication affects the clinical outcomes of anecortave acetate for depot suspension in the treatment of patients with exudative agerelated macular degeneration (AMD). ARVO Abstract #2366, 2005.
22. Russell SR, Slakter JS, Ho AC, et al. Anecortave acetate treatment of dry AMD to reduce risk of progression to wet AMDthe Anecortave Acetate Risk Reduction Trial (AART). ARVO Abstract #3134, 2004.
23. Garcia CA, QuirozMercado H, Uwaydat S, et al. A Phase I/II trial of intravenous squalamine lactate for treatment of choroidal neovascularization in age related macular degeneration (ARMD). ARVO Abstract #2362, 2004.
24. Kupperman BD, Blumenkranz MS, Fribert TR, et al. Advances in AMD Therapy. Rev Ophth 2005 May;12(5):98a-98p.
25. Rosenfeld PJ, Puliafito CA, Michels S, et al. Systemic Bevacizumab (Avastin) Therapy for Neovascular AgeRelated Macular Degeneration (SANA) Study: visual acuity outcomes. ARVO Abstract #2310, 2005.
26. Balaggan KS, Binley K, Esapa M, et al. EIAV vector delivery of angiostatin or endostatin inhibits angiogenesis and vascular hyperpermeability in experimental CNV. ARVO Abstract #4694, 2005.
27. Bouhana K, Gomez A, Fosnaugh K, et al. Stabilized small interfering RNAs (siRNAs) targeting the vascular endothelial growth factor (VEGF) pathway inhibit angiogenesis in vivo in a rat corneal model. ARVO Abstract #4822, 2004.
28. Yang Y, Meadows S, Li PK, et al. Anti-angiogenic effect of linomide analogue. ARVO Abstract #4166, 2005.
29. Zahn G, Stragies R, Wills M, et al. Evaluation of small molecule integrin inhibitor for ocular neovascularization in laser induced CNV model in monkey. ARVO Abstract #4169, 2005.
30. Ramakrishnan V, Kuppermann BD, Bhaskar V, et al. F200, a Fab derivative of M200 (volociximab; antiA51), is a potent inhibitor of angiogenesis in a rabbit model of choroidal neovascularization. ARVO Abstract #465.
31. Wen R, Zhao L, Liu Y, et al. VEGF trap induces significant regression of existing choroidal neovascularization (CNV). ARVO Abstract #5307, 2005.
32. Takahashi K, Saishin Y, Silva RL, et al. Systemic or intraocular administration of VEGF receptor kinase inhibitors causes regression of choroidal neovascularization. ARVO Abstract #1423, 2005.
33. Song SJ, Kim K, Kim Y, et al. Inhibitory effect of YC-1 on experimental choroidal neovascularization in rat. ARVO Abstract #1418, 2005.
34. Akiyama H, Silva RL, Kachi S, et al. A VEGF121gelonin fusion protein (VEGF121/rGel) induces regression of subretinal and choroidal neovascularization (CNV). ARVO Abstract #455, 2005.

