Second only to uncorrected refractive error among causes of visual impairment, cataract is the number one cause of preventable blindness worldwide.1 Cataract can occur at all ages due to a multitude of precipitating events, but of course age-related cataract comprises the vast majority of cases. Although the scenario is less common, early-onset lens disease can affect functional vision to the point where patients require ophthalmic intervention much earlier in life. These non-age-related changes of the crystalline lens can be classified as congenital or infantile (present within the first year of life), juvenile (within the first decade of life) or presenile cataract (present before the age of about 45 years).3
While the management approach of surgical extraction and IOL implantation will not differ in principle from that used in age-related cataract, identifying the form and cause may uncover treatable systemic diseases that require attention.
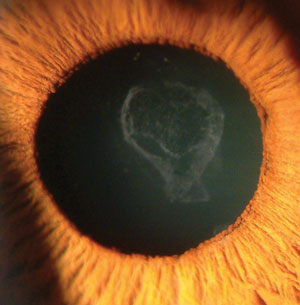 | |
| Diabetic cataract—the result of uncontrolled diabetes—located in the region of the anterior lens cortex. |
Why So Soon?
Risk factors associated with development of presenile cataracts include gender, race, exposure to UV radiation, smoking, genetic predisposition, diet, body mass index, systemic diseases, medications, ocular trauma and secondary ophthalmic disease.4-13 While age-related cataracts are an asymmetric phenomenon, cataracts of non-age-related etiologies are often either asymmetric or unilateral.4-13
Ocular diagnoses associated with secondary cataract may include: uveitis, glaucoma, retinal detachment, retinal degenerations such as retinitis pigmentosa or gyrate atrophy, persistent hyperplastic primary vitreous (PHPV), aniridia, Reiter’s anomaly, sclerocornea, micro-ophthalmia, retrolenticular fibroplasia, Norrie’s disease, anterior segment degeneration and necrosis, retinoblastoma and high myopia.3,12,13 Other causes include general atopy, high myopia, steroid intake, sunlight exposure and diabetes.6-13 Another risk factor is vitreoretinal surgery of any kind with virtually a 100% chance of lens opacity associated with vitretomy.15-17
Many ophthalmic surgical procedures performed on the anterior and posterior segment of the eye result in the formation of cataract. Glaucoma procedures have long been recognized to result in cataractogenesis, which was revealed in the Collaborative Initial Glaucoma Treatment Study (CIGTS).14
Posterior segment procedures, including the injection of medications and invasive surgery such as vitrectomy or repair of retinal detachment can be associated with the eventual formation of cataract.15-17
Anatomy and Physiology
The anatomical nature and position of the crystalline lens within the eye makes any abnormality in its structure capable of producing a deficiency in its function.1,2,18-24
Bringing Clarity to ‘Cataract’
The amount of visual disability produced is related to the location (central or peripheral, anterior or posterior), the size and the nature (cortical, nuclear, subcapsular) of the opacity, as well as the visual demands of the individual. Symptoms can range from visual dimming to difficulties in low levels of illumination. Noticeable alterations in the colors of things, disability glare and decreased visual acuity both at distance and at near are common. |
The structure, via an encircling network of zonules that connect the supportive surrounding capsule to the circumferential ciliary muscle and gland, enables the focusing of images onto the retina. Any irregularity in lens tissue architecture will lead to zones of opacity in the lens. Depending on where they are, their density and their linear area, variable symptoms such as glare and loss of acuity occur. The lens is underpinned by specific developmental physiology established prenatally during organogenesis. During development, surface ectoderm associated with the neural retina invaginates to form the lens vesicle.18,19 Cells in the posterior half of the lens differentiate into primary lens fibers that form the lens fiber core. Those in the anterior half maintain a proliferative state as a monolayer of lens epithelium.18 Differentiating lens fiber cells elongate and cover the old lens fiber core, resulting in spatially-regulated growth of the lens during development.18
As the lens forms, its various central regions are referred to as nuclei. The lens has four naturally occurring nuclei: the embryonic nucleus, the fetal nucleus, the juvenile nucleus and the adult nucleus. The differing regions of the lens meld together, forming two Y-shaped sutures, visible upon biomicroscopy.3 A “hinged” design permits the structure to change shape by altering its convexity, while maintaining relative clarity and superior optical properties (ciliary body contraction induces slack on the zonules, creating more convexity and increased plus power, while relaxation of the ciliary body tightens the zonules to create less convexity and hence less plus power).2,18
Congenital Cataract
Conditions present at birth account for one in 10 childhood blindness cases, and approximately half of congenital cataracts are genetically determined.8,25 The prevalence of cataract in childhood is estimated between one and six per 10,000.25 Of patients diagnosed with lens disease in their first year of life, more than 8% had a positive family history of cataract. Of the children with a positive family of history of congenital cataract, 8% presented with unilateral involvement.25
Congenital cataracts may be isolated, or can be associated with other developmental abnormalities of the eye.8 They may also be associated with inherited multi-system disorders.25 Causes of congenital cataracts include intrauterine infections (rubella, varicella, toxoplasmosis), metabolic disorders, trauma and juvenile ocular inflammatory disease.25 In many instances, especially in unilateral presentations, the etiology of the cataract is unknown.25
In one study evaluating idiopathic cases, 67% of the cases were characterized by a history of prenatal maternal illness. The remaining 22% involved the use of medication during pregnancy.25 In the United States, routine immunization has rendered cataract from congenital rubella virtually nonexistent.26
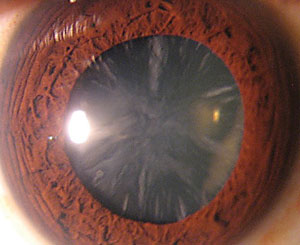 | |
| Stellate cataract of the anterior lens cortex as a result of phenothiazine use. |
Most congenital cataracts are inherited as an autosomal dominant trait.3,4 Much like senile cataracts, childhood cataracts are categorized according to both their appearance and distribution of opacification.3,4 The opacification nomenclature includes nuclear, lamellar, pulverulent, posterior polar, cerulean (blue dot) and anterior polar opacities.26 Polar opacities involve either the anterior or posterior pole of the lens (or both, referred to as bipolar opacities), as well as posterior subcapsular cortex opacities. Zonular cataracts include specific lens regions (including nuclear cataracts, which affect the fetal or embryonic nucleus, and lamellar cataracts).26 Membranous or capsular cataracts can result from resorption of lens proteins after capsular rupture.26 In membranous cataracts, the early lens was traumatized or experienced severe dysfunction.26
Pharmacologic Cataract
The toxic effects of medications on the lens manifest as cloudiness in different anatomical locations, determined by the route of drug administration.11 For example, equatorial lens changes are common with systemically administered drugs that cross the blood-aqueous barrier.11 Centrally located anterior lens changes are commonly associated with use of topically administered drugs.11 Posterior subcapsular and cortical changes occur secondary to the diffusion of noxious substances from the posterior chamber crossing the blood/aqueous barrier.11 Uveal inflammation and disturbances within the vitreous humor can produce these as well.11
We commonly see posterior subcapsular cataracts in patients who have been prescribed oral, topical, inhaled or injectable corticosteroids for long periods of time.11 Intravitreal injections of triamcinolone used in the treatment of retina pathology is implicated in the development of cataract, particularly posterior subcapsular changes. After a single injection, upwards of 15% to 20% of patients developed symptomatic cataract that warranted extraction. In patients who received multiple injections, 70% developed symptomatic cataract, including posterior subcapsular, nuclear and cortical varieties.27,28
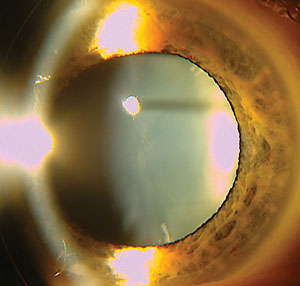 | |
| Congenital anterior polar cataract located on the anterior capsular surface. |
Although the exact mechanism of steroid-induced cataract remains unknown, it is likely that glucocorticoid-induced gene transcription events in lens epithelial cells and also other intraocular or systemic cells interact to generate steroid-induced cataracts.29 The incidence of steroid-induced cataract is related to the dosage and duration of both topical and oral steroid administration.
Steroid cataractogenesis begins in the posterior pole as refractile or multicolored dots that gradually evolve, making it difficult to differentiate from other cataracts. This characteristic can be used to detect the cataract formation early in the course of chronic steroid use. Lens opacities caused by corticosteroids may progress or remain stationary, but rarely regress upon withdrawal of the corticosteroid.11
The psychotropic medications chlorpromazine and thioridazine are common phenothiazines involved in cataract formation.11 Both are used in the treatment of depressive or organic psychoses and schizophrenia.11 Use of these medications use may lead to the accumulation of fine, white to yellowish-brown granules in the anterior cortex, as well as beneath the anterior capsule and in the area of the pupillary aperture.11 As the opacity progresses, a stellate pattern of granules develops, becoming a true anterior polar cataract.11
Antineoplastic agents used to treat leukemia and other blood dyscrasias are associated with the development of posterior subcapsular lens opacities.11 These lens changes present as scattered punctate cortical opacities and posterior lens capsule polychromatic sheen. It is thought that cataractogenesis is related to interference with nucleic acid metabolism during mitosis in the cell of the lens epithelium.11
Traumatic Cataract
Ocular trauma, resulting in permanent vision loss in 2% to 14% of children, is a leading cause of acquired monocular blindness, and may be the result of penetrating injury, concussive force, electric shock or radiation.30 Open globe injuries have the most significant association with visually debilitating cataract.30-35 Anatomically, younger patients exhibit stronger adherence between the posterior lens capsule and anterior vitreous.30 Furthermore, the tertiary vitreous is anatomically connected to the peripheral retina at the vitreous base. Traction on the vitreous face transmitted to the retina and lens creates risk for both posterior segment disease and cataract formation.30 In cases of penetrating and concussive force injuries, cataract formation generally occurs in the anatomical region of the lens where the trauma had induced traction on the lens. All regions of the lens have the potential to be associated with traumatic cataract, because any region is subject to trauma.30-32
| ‘Typical’ Cataract Formation Age-related cataracts are produced when insoluble lens crystallin proteins and other fibrillary materials aggregate within the lens stroma.19-22 Small molecules known as mini-chaperones reduce protein aggregation by maintaining activity, which reduces the aggregation of proteins by blocking amyloid fibril formation, stabilizing mutant proteins, sequestering metal ions and exhibiting antiapoptotic properties.19,20 The natural extinction of these chaperone proteins, due to their finite number within the lens, leads to the accumulation of protein and fibril aggregation.19-22 Advanced glycation end-products (AGE) have also been implicated in cataract formation.23,24 The progressive accumulation of AGE in lens tissue mediates irreversible changes in structural proteins, provoking the formation of high molecular weight aggregates that scatter light and impede vision.24 Further, AGE are responsible for creating aberrant crosslinking of extracellular matrix proteins, disrupting endothelial junctional complexes and creating areas of opacification and clefting.22-24 In a process involving oxidative radicals, the lens naturally undergoes architectural changes, which produce the precursor opacities to mature cataract. |
The incidence of cataract resulting from electrocution varies greatly, due to differences in voltage and duration of contact with the current.33 In addition, the distance from the site of electrocution to the eye, extent of surface contact and the direction the current took through the body all contribute to the type and amount of cataract development.33 The onset of cataract development following electrocution may occur within several days, or upwards of 18 months subsequent to the initial injury.33 Early electrocution lens changes appear as a collection of multiple fine vacuoles below the anterior capsule in the midperiphery of the lens. This may require dilation to detect. Over time, the vacuoles progress to flake-like opacities, coalescing and migrating into the line of vision.33 Although this type of cataract is often bilateral, the formation of the initial cataract will occur in the eye closest to the site of contact.33
Radiation cataracts develop six months to one year following local or systemic exposure. Late-onset cataracts have been reported decades following exposure.35,36 The most common site of cataract following radiation injury is the posterior subcapsular region of the lens. Opacification is due to radiation damage to the lens epithelium around the equator, which disrupts epithelial DNA, causing abnormal differentiation of the elongating fibers. These cataracts often migrate into the posterior subcapsular area.34-36
Metabolic Cataract
Metabolic syndrome (MetS) represents a cluster of metabolic abnormalities involving central obesity, dyslipidemia, hyperglycemia and high blood pressure.37,38 Studies show an association between MetS and an increased risk of developing cortical, nuclear and posterior subcapsula cataract subtypes.39-41
Compared with the general population, patients with diabetes tend to develop cataracts at an earlier age.42,43 They also have a propensity to progress more quickly. Visual symptoms commonly occur as a result of secondary myopia, with hyperosmotic lens changes secondary to hyperglycemia.42,43 Often, cataracts noted in young diabetes patients present as diffuse posterior and anterior subcapsular or cortical snowflake opacities.42,43 Although the pathogenesis of the diabetic cataract is not completely understood, many theories exist.42-44
Poor blood sugar control results in hyperglycemia, which is associated with the conversion of glucose to sorbitol in the polyol pathway. Accumulation of sorbitol in the lens occurs for two main reasons:
- Sorbitol is produced faster than it is converted to fructose; an enzyme called sorbitol dehydrogenase is at low ocular concentrations.
- The enzymes that compete to turn glucose into sorbitol or fructose don’t have the same “strengths” or activities.
- Sorbitol is precluded from diffusing through the aqueous/blood barrier due to its classification as a sugar alcohol—its polarity hampers its ability to pass through the lipophilic blood/aqueous barrier.
For these reasons, sorbitol concentration supersedes the concentration of fructose.42-44 The accumulation of sorbitol creates a hyperosmotic effect (e.g., it changes the tonicity of the ocular tissue relative to its surroundings, drawing in water). Hyperosmotic stress created by sorbitol accumulation induces apoptosis of lens epithelial cells.42-44
Central obesity, dyslipidemia and high blood pressure (and medical therapies used to treat these diagnoses) all have an association with the early development of cataract, particularly in cases where the diagnosis is uncontrolled.42,43 Lens opacification may occur at the level of the cortex, nucleus and posterior subcapsular region, or may present in all three levels simultaneously.42,43
Treatment
Early detection and management of cataract in young patients avoids amblyopia and provides the best opportunity for optimal visual function throughout life. The evolution of surgical techniques, intraocular lens composition and design, along with a greater understanding of the neural biology of both visual development and early postoperative optical rehabilitation, all play a role in improving outcomes.46,47
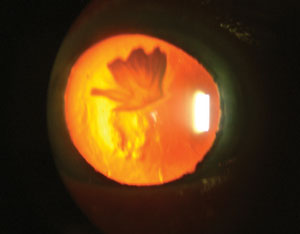 | |
| Partial rosette cataract involving the anterior cortex of the lens, imaged using retroillumination. |
The anatomy of a child’s eye poses a unique challenge for the pediatric cataract surgeon. The young eye has a shorter mean axial length, a steeper cornea, a thin and less rigid sclera and a more elastic lens capsule, putting it at greater risk of developing a severe inflammatory response following surgery.46,47 Because of the growth of the child’s eye, the preoperative biometry for intraocular lens calculation can be quite complex. With this in mind, and because of myopic shift as the eye grows in axial length, the selection of the intraocular lens power must be suited for not only the acute postoperative period, but also for future visual function. Adding to the difficulties, a young child may not cooperate while the clinician attempts to obtain accurate axial length and keratometry readings.46,47
Even when considering the above, a recent survey concluded that pediatric ophthalmologists in the United States prefer implanting intraocular lenses at the time of cataract surgery.46,48 If the decision is made not to implant the lenses, both aphakic spectacles and contact lenses may maximize best corrected visual acuity, reducing the likelihood of the development of amblyopia.
Depending on the age of the child, initial spectacle design may or may not include a bifocal power. Management of residual refractive error with contact lens wear may consist of a soft or rigid gas permeable lens. Lens wear is generally well tolerated and provides flexibility in the lens power as the visual system develops.
Postoperative care may include vision rehabilitation aimed at reducing the potential development of amblyopia. Therapeutic activities consistent with the recommendations of the National Eye Institute’s Amblyopia Treatment Study I and II, such as direct occlusive therapy with a patch or atropine drops and organized binocular visual tasks (vision therapy), have been included in the postoperative course of pediatric cataract management.46,48
In cases involving young adults rather than infants and adolescents, concerns with respect to the developmental anatomy of the eye are not as great.46,47 Such patients cooperate during measurements, and the likelihood of permanent degradation of best-corrected visual acuity is minimal. These patients generally have intraocular lenses implanted, with postoperative residual refractive error corrected with either spectacles or contact lenses.46,47
Any interruption in the structure, architecture or physiology of the crystalline lens can induce the formation of visually significant opacities capable of impacting function and lifestyle. Patients are vulnerable at any age. While we all will succumb to the age-related processes of opacification, presenile and other early-onset cases typically are accompanied by other health concerns that require care with the appropriate specialist.
Optometrists are uniquely positioned to be the first medical professionals to identify these cases and coordinate care as needed. Early diagnosis and management, thanks to modern materials and techniques, can provide solutions that mitigate amblyopia and provide excellent long-lasting outcomes for our patients.
Dr. Gurwood is professor of clinical sciences at The Eye Institute at Salus University in Philadelphia.
Dr. Myers is senior staff optometrist at the Coatesville Veterans Affairs Hospital in Pennsylvania.
1. Dobrzynski JM, Kostis JB. Statins and cataracts—a visual insight. Curr Atheroscler Rep. 2015;17(2):477.2. Merriam Webster. Cataract. www.merriam-webster.com/dictionary/cataract.
3. Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19(2):134-49.
4. Ketibeh M, Eskandari A, Yaseri M, et al. The gender issue in congenital and developmental cataract surgery. J Ophthalmic Vis Res. 2013;8(4):308-13.
5. Prakalapakorn SG, Rasmussen SA, Lambert SR, Honein MA. The national birth defects prevention study. Ophthalmology. 2010;117(8):1500-15.
6. Kleiman NJ. Radiation cataract. Ann ICRP. 2012;41(3-4):80-97.
7. Kar T, Ayata A, Aksoy Y, et al. The effect of chronic smoking on lens density in young adults. Eur J Ophthalmol. 2014;24(5):682-7.
8.Francis PJ, Moore AT. Genetics of childhood cataract. Curr Opin Ophthalmol. 2004;15(1):10-5.
9. Weintraub JM, Willett WC, Rosner B, et al. A prospective study of the relationship between body mass index and cataract extraction among US women and men. Int J Obes Relat Metab Disord. 2002;26(12):1588-95.
10. Salowi MA, Goh PP, Lee MY, et al. The malaysian cataract surgery registry: Profile of patients presenting for cataract surgery. Asia Pac J Ophthalmol (Phila). 2015;4(4):191-6.
11. Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Safety. 2008;31(2):127-41.
12. Praveen MR, Shah GD, Vasavada AR, et al. A study to explore the risk factors for the early onset of cataract in India. Eye. 2010;24(6):686-94.
13. Vasudevan M, Premnath G. A prospective observational study to analyze the causes and types of presenile cataract in South Indian patients. J of Evolution of Med and Dent Sci. 2014;53(3):12308-15.
14. Mochizuki T, Masai I. The lens equator: A platform for molecular machinery that regulates the switch from cell proliferation to differentiation in the vertebrate lens. Dev Growth Differ. 2014;56(5):387-401.
15. Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280(1):1-14.
16. Raju M, Santhoshkumar P, Krishna SK. Alpha-crystallin-derived peptides as therapeutic chaperones. Biochim Biophys Acta. 2015;pii:S0304-4165(15)00172-5.
17. Raju M, Santhoshkumar P, Sharma KK. αA-Crystallin-derived mini-chaperone modulates stability and function of cataract causing αAG98R-crystallin. PLoS One. 2012;7(9):e44077.
18. Cetinel S, Unsworth L, Montemagno C. Peptide-based treatment strategies for cataract. J Glaucoma. 2014;23(8 Suppl 1):S73-6.
19. Kandarakis SA, Piperi C, Topouzis F, Papavassiliou AG. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog Retin Eye Res. 2014;42:85-102.
20. Nagaraj RH, Linetsky M, Stitt AW. The pathogenic role of maillard reaction in the aging eye. Amino Acids. 2012;42(4):1205-20.
21. Yi J, Yun J, Li ZK, et al. Epidemiology and molecular genetics of congenital cataracts. Int J Ophthalmol. 2011;4(4):422-32.
22. Papania MJ, Wallace GS, Rota PA, et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the western hemisphere: the US experience. JAMA Pediatr. 2014;168(2):148-55.
23. Jonas JB. Intravitreal trimamcinolone acetonide: a change in paradigm. Ophthalmic Res. 2006;38(4):218-45.
24. Cekic O, Chang S, Tseng JJ. Cataract progression after intravitreal triamcinolone injection. Am J Ophthalmol. 2005;139(6):993-8.
25. Kanthan GL, Wang JJ, Rochtchina E, Mitchell P. Use of antihypertensive medications and topical beta-blockers and the long-term incidence of cataract and cataract surgery. Br J Ophthalmol. 2009;93(9):1210-4.
26. Zhu L, Wu Z, Dong F, et al. Two kinds of ocular trauma score for pediatric traumatic cataract in penetrating eye injuries. Int J Care Injured. 2015;46(11):1828-33.
27. Khokhar S, Gupta S, Agarwal T. Epidemiology and intermediate-term outcomes of open- and closed-globe injuries in traumatic childhood cataract. Eur J Ophthalmol. 2014;24(1):124-30.
28. Lee SI, Song HC. A case of isolated posterior capsule rupture and traumatic cataract caused by blunt ocular trauma. Korean J Ophthalmol. 2001;15(1):140-4.
29. Hashemi H, Jabbarvand M, Mohammadpour M. Bilateral electric cataracts: Clinicopathologic report. J Cataract Refract Surg. 2008;34(8):1409-12.
30. Grewal DS, Jain R, Brar GS, Grewal SPS. Unilateral electric cataract: Scheimpflug imaging and review of the literature. J Cataract Refract Surg. 2007;33(6):1116-9.
31. Mohammadpour M, Movaheden ZE, Jabbarvand M, Hashemi H. Radiation cataract: Clinicopathologic report. J Cataract Refract Surg. 2013;39(2):285-8.
32. Wilde G, Sjöstrand J. A clinical study of radiation cataract formation in adult life following ɣ radiation of the lens in early childhood. B J Ophthalmol. 1997;81(2):261-6.
33. Maralani HG, Tai, BC, Wong TY, et al. Metabolic syndrome and risk of age-related cataract over time: An analysis of interval-censored data using a random-effects model. Invest Ophthalmol Vis Sci. 2013;54(1):641-6.
34. Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(3):469-80.
35. Tan JS, Wang JJ, Mitchell P. Influence of diabetes and cardiovascular disease on the long-term incidence of cataract: The Blue Mountains Eyes Study. Ophthalmic Epidemiol. 2008;15(2):317-27.
36. Sabanayagam C, Wang JJ, Mitchell P, et al. Metabolic syndrome components and age-related cataracts: The Singapore Malay Eyes Study. Invest Ophthalmol Vis Sci. 2011;52:2397-404.
37. Bojarskiene F, Cerniauskiene LR, Paunksnis A, Luksiene DI. Association of metabolic syndrome components with cataract. Medicina. 2006;42(1):115-22.
38. Al-Agha A, Ocheltree A, Rashad R, Abdelsalam I. Metabolic cataract in an 8-year-old diabetic boy. Turk J Pediatr. 2012;54(1):83-5.
39. Nabarro JD. Diabetes and United Kingdom: A personal series. Diabetic Med.1991;8(1):59-68.
40. Pollreisz A, Schmidt-Erfurth U. Diabetic cataract—pathogenesis, epidemiology and treatment. J Ophthalmol. 2010 June 17. [Epub].
41. Medsinge A, Nischal KK. Pediatric cataract: Challenges and future directions. Clin Ophthalmol. 2015;9(1):77-90.
42. Wilson ME, Trivedi RH, Morrison DG, et al. Infant aphakia treatment study: Evaluation of cataract morphology and eyes with monocular cataracts. J AAPOS. 2011;15(5):421-6.
43. Wilson ME, Triverdi RH. Choice of intraocular lens for pediatric cataract surgery: Survey of AAPOS members. J Cataract Refract Surg. 2007;33(9):1666-68.
44. Pollreisz A, Schmidt-Erfurth U. Diabetic cataract - pathogenesis, epidemiology and treatment. J Ophthalmol 2010; Epub 2010 Jun 17.
45. Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44(3):155-65.
46. Medsinge A, Nischal KK. Pediatric cataract: Challenges and future directions. Clin Ophthalmol 2015;9(1):77-90.
47. Wilson ME, Trivedi RH, Morrison DG, Lambert SR, Buckley EG, Plager DA, Lynn MJ. Infant Aphakia Treatment Study Group. Infant aphakia treatment study: Evaluation of cataract morphology and eyes with monocular cataracts. J AAPOS. 2011;15(5):421-6.
48. Wilson ME, Triverdi RH. Choice of intraocular lens for pediatric cataract surgery: Survey of AAPOS members. J Cataract Refract Surg. 2007;33(9):1666-68.

