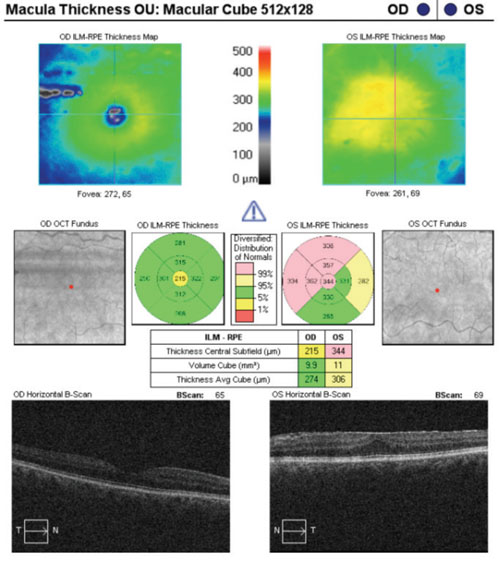
 |
| Ganglion cell analysis complicated by the presence of an epiretinal membrane. |
Over the past several years, SD-OCT has amassed a huge and growing popularity in eye care (and rightfully so). There is no necessary preparation of the tissue being imaged, and very little is required on the part of the patient. The ability to better detect and stage macular edema, along with the ability to examine specific layers of the retina, has proven to be highly beneficial in the care of our patients. Furthermore, SD-OCT arrived on the scene at an opportune time, as the baby boomers are at a point in life at which corneal, lenticular, macular and optic nerve issues are more commonplace.1
SD-OCT may be well on its way to becoming standard of care for diseases such as glaucoma. On one hand, detectable structural damage precedes detectable functional damage most of the time.2 One reason for this may be the presence of overlap in visual receptor fields. Another reason may be that the perfect visual field study just doesn’t seem to exist. SD-OCT has already proven to be highly valuable at determining the presence and rate of structural progression.3 Image registration has proven to be highly beneficial in this capacity.
On the other hand, many patients who need visual field studies (which are still very much standard of care for glaucoma) have cognitive or physical impairments, or both. Combine this with the high level of subjectivity that accompanies a visual field study, and it becomes obvious why the clinician may, at times, be left with less-than-definitive information.
Have I convinced you yet as to why I tuck my SD-OCT in and read it a bedtime story every night when I leave my office?
The Art of Artifact
SD-OCT is highly valuable and quite fun, but there are two things it just doesn’t do: think and diagnose. Further, it doesn’t directly measure the tissues of the human eye. It instead measures differences in the light reflectivity of those tissues.4 The various tissues, and layers of tissue, in the eye reflect light at different levels, so they show up as distinct entities on an OCT study. However, these different levels of reflectivity do enable the instrument to segment the various components of the tissue being imaged.
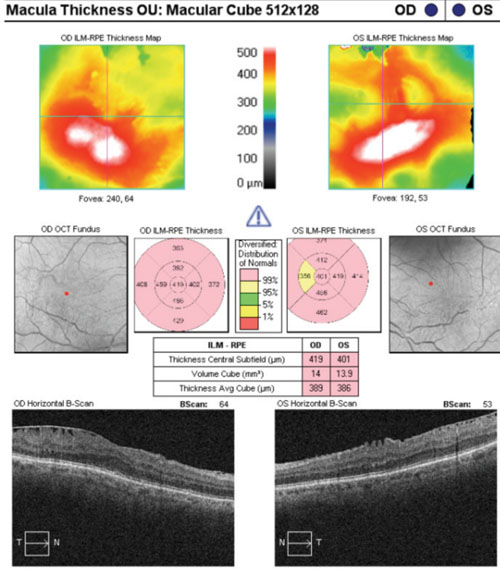 |
 |
| Ganglion cell analysis rendered essentially useless due to epiretinal membranes. |
For example, the retinal nerve fiber layer (RNFL) is highly optically reflective, while the vitreous humor exhibits a relatively low level of reflectivity (you might call it “optically quiet”). These differences in reflectivity that correspond to the different tissues of the human eye work in favor of the clinician’s interpretation most of the time.
However, lurking variables do exist and may lead to misinterpretation if not accounted for.
The presence of artifact is well known to skew interpretations of imaging studies, and SD-OCT is certainly not immune to this concept. In fact, a recent study looking at SD-OCT imaging for glaucoma showed artifacts to be present anywhere from 15% to 36% of the time.5
Common artifacts include epiretinal membranes, tractional vitreomacular adhesions, eye movements and media opacities. Normal retinal vasculature may act as artifact, as well.6
• Epiretinal membranes. Epiretinal membranes may not always be obvious when viewed with a precorneal lens, and are certainly less obvious via retinal photography. However, they are often seen more easily on SD-OCT studies. An instance comes to mind in which I happened upon an epiretinal membrane by chance while looking at an OCT printout; I had failed to pick it up during funduscopy. It only takes a mild epiretinal membrane to distort the underlying inner retina enough to affect the values of an SD-OCT study, such as ganglion cell analysis.
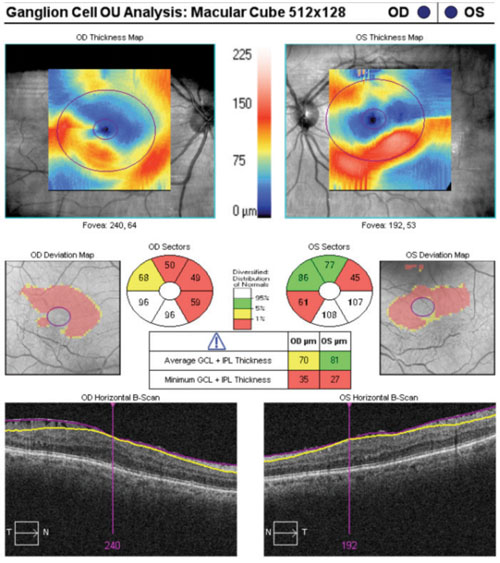
Case in point: A 66-year-old white female presented for a glaucoma work-up and was found to have bilateral epiretinal membranes. The epiretinal membrane on the left macula severely skewed the ganglion cell analysis. In a similar case of a 65-year-old white male, bilateral epiretinal membranes acted as artifacts, which essentially rendered the ganglion cell analysis useless.
Epiretinal membranes reflect light in a similar fashion to the innermost layers of the retina (namely the internal limiting membrane). At the very least, they can distort the thickness values of the inner retina. And, although such distortion may only be miniscule, given the high resolution of SD-OCT technology, even a few microns matter. Careful examination of macular studies, in addition to the ganglion cell analysis printout, can aid the astute clinician in avoiding this error.
• Uncommon vasculature. Anomalous physiologic findings such as epiretinal membranes and vitreomacular adhesions from anomalous posterior vitreous detachments may, at times, have a propensity to skew SD-OCT values with the potential to lull the clinician into a false sense of security or make him or her lean toward a diagnosis that may not be present.
However, congenital findings or “variants on normal” may be misleading, as well. Take this case for instance: An 18-year-old black male was referred to our office as a glaucoma suspect due to enlarged optic cups. He was mildly myopic with a history that was otherwise noncontributory. Over three visits at three different points in the day, his highest recorded intraocular pressure was 16mm Hg OU. His anterior segments and angles were unremarkable, with no signs of dysgenesis. His SD-OCT studies reflected wedge-shaped areas of his RNFL supero-temporally OU that fell outside of the normative database for his age. At first glance, these wedge-shaped “defects” appeared to resemble glaucomatous RNFL loss.
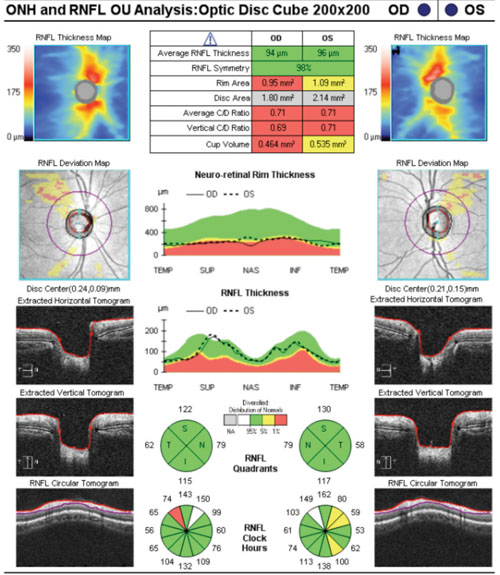 |
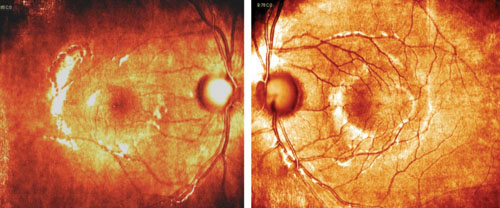 |
| Nasalization of retinal vasculature, with corresponding RNFL nasalization, causes points to be flagged in areas suspicious for glaucomatous damage on the RNFL deviation map. |
The relative symmetry of this patient’s RNFL also points to a variant on normal rather than the presence of pathology. Tilted optic discs often show a similar appearance on OCT studies. Such cases exemplify how normative databases can be both good and not-so-good at the same time. If we take the “stoplight” approach to the OCT printout—green means go (monitor) and red means stop (treat)—then we would end up incorrectly treating this patient as having glaucoma, and not as a false positive (which is the correct diagnosis).
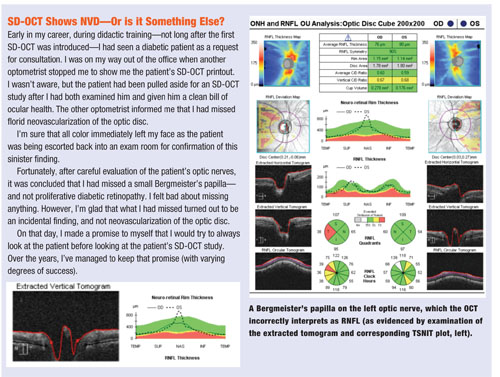
• Bergmeister’s papilla. Another finding that can be overlooked is a subtle Bergmeister’s papilla—a small, fibrous tuft that represents a remnant of the hyaloid artery. Bergmeister’s papillae, if significant enough, could be mistaken as RNFL, potentially misleading the clinician on interpretation of an SD-OCT optic nerve study.
The TSNIT curve and corresponding tomogram—two areas of the SD-OCT printout that may be overlooked—can indicate the presence of potentially lurking variables, such as subtle Bergmeister’s papillae or epiretinal membranes.
SD-OCT technology has truly given us the benefit of quantifying disease processes down to just a few microns (and with little to no effort on the part of the patient). It has augmented how we think about and diagnose several diseases. It will only grow more commonplace as more clinicians make use of it and improvements continue to be made to its already incredible (and incredibly fun) functionalities. In addition, advances in the technology may someday allow us to use SD-OCT in place of invasive diagnostic tests, such as fluorescein angiography studies, for certain retinal and optic nerve conditions.7
However, SD-OCT technology, as sophisticated as may be, will never yield the same qualitative information as actually looking directly at the tissues of the human eye. For instance, we diagnose people as glaucoma suspects because we’ve determined that their optic nerves look suspicious for glaucoma. (This is why there is a separate ICD code for “ocular hypertension” that is different from the ones for “glaucoma suspect.”) We then employ the use of tests such as gonioscopy, pachymetry, visual fields and SD-OCT studies to help confirm or deny what we suspect. However, the process for diagnosing disease should, when at all possible, begin with direct evaluation of the tissue in question.
So, SD-OCT technology is an excellent way to achieve objective information (both quantitative and qualitative) in the presence of diseases such as age-related macular degeneration and glaucoma.
However, confounding variables do exist, and it’s our responsibility to discern and account for their presence.
Refer for OCT If Need Be
Because SD-OCT testing is so valuable, many think that this technology should be made available to all patients who may benefit from it. If you haven’t yet acquired the technology, but your patient requires SD-OCT, you can still give the patient access to SD-OCT—and you can remain in charge of the patient’s care—by means of a referral for testing.
When referring out, the physician who performs the OCT never examines the patient, but only performs the test (and bills one of the OCT codes: 92132, 92133 or 92134, with the -TC modifier for the testing component). The test results return to you, the referring physician, so you can interpret the results and explain them to the patient (and bill for the professional component using the same OCT code along with the -26 modifier).
Dr. Casella is a third-generation optometrist who practices in Augusta, Ga., with an emphasis on ocular disease. He was recently named SECO’s Young Optometrist of the South.
1. Seddon JM. Genetic and environmental underpinnings to age-related ocular diseases. Invest Ophthalmol Vis Sci. 2013 Dec 13;54(14):ORSF28-30.
2. Hood DC, Karden RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007 Nov;26(6):688-710.
3. Leung CK. Diagnosing glaucoma progression with optical coherence tomography. Curr Opin Ophthalmol. 2014 Mar;25(2):104-11.
4. Arevalo JF, Krivoy D, Fernandez CF. How Does Optical Coherence Tomography Work? Basic Principles. In: Arevalo JF, ed. Retinal Angiography and Optical Coherence Tomography. New York, NY: Springer; 2009:217-22.
5. Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol. 2014 Feb 13. [Epub ahead of print.]
6. Balk LJ, Mayer M, Uitdehaag BM, Petzold A. Retinal hyperaemia-related blood vessel artifacts are relevant to automated OCT layer segmentation. J Neurol. 2014 Mar;261(3):511-7.
7. Hutchinson JK, Street M, Gurwood AS, Myers MD. Face Off: OCT vs. Fluorescein. Rev Optom. 2011 Sep;148(9):62-75.

