 A 56-year-old white male was being evaluated for glaucoma. All of the necessary testing was performed. His central corneal thickness measured 570µm O.U. Gonioscopically, his anterior chamber angles were open, without any abnormalities.
A 56-year-old white male was being evaluated for glaucoma. All of the necessary testing was performed. His central corneal thickness measured 570µm O.U. Gonioscopically, his anterior chamber angles were open, without any abnormalities.
His optic nerves showed notching of the neuroretinal rim and moderate glaucomatous changes in both eyes. His visual field manifested arcuate scotomas with non-threatening fixation.
His intraocular pressure (IOP) measured 30mm Hg O.D. and 31mm Hg O.S. Clearly, the man had primary open-angle glaucoma in each eye and, after a discussion of the disease and risks and benefits of treatment, it was decided that he should begin medical therapy to reduce IOP.
We chose a prostaglandin analog for therapy. However, the question was whether to employ the monocular trial and use the medication initially in one eye while leaving the fellow eye temporarily untreated as a control in order to judge the efficacy of the medication, or simply to prescribe the medication bilaterally. In this month’s column, we examine the scientific rationale behind a monocular trial.
What is a Monocular Trial?
It has been long advocated that, when initiating medical pressure reduction for patients with glaucoma, a short trial of the medication should be used in one eye only, leaving the fellow eye untreated (assuming no significant risk of vision loss exists) to serve as a control.1
In a monocular trial, the patient uses the medication unilaterally until the agent achieves a constant effect. The argument for using monocular trials is that IOP is variable—both in healthy individuals and in those with glaucoma. By initially treating only one eye and using the other as an untreated control, it has been postulated that the IOP reduction in the treated eye will reflect both the therapeutic effect of the drug and the spontaneous change; whereas the fellow eye will exhibit only the spontaneous change in IOP.
Theoretically, the monocular trial should minimize the impact of spontaneous change in IOP and highlight the drug’s therapeutic response. Thus, if a therapeutic effect is determined in one eye, the fellow eye will likely demonstrate a similar therapeutic response. Then, bilateral treatment can be initiated.
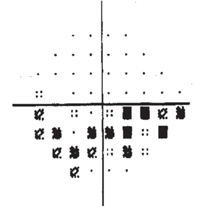
Evidence of an arcuate defect that was documented in a patient with glaucoma. Should we initiate a monocular trial in this individual?
In most instances, the decision about a medication’s efficacy is determined by the monocular trial, and is based upon the difference between one pre-treatment and one post-treatment IOP measurement.
Assumptions
While the aforementioned arguments for a monocular trial seem reasonable, they are based upon several assumptions:
• The diurnal variation in IOP is similar between both eyes in a single patient.
• The diurnal variation is similar both in the short and long term.
• The therapeutic response in one eye will be identical to that seen in the fellow eye.
Analyzing the Assumptions
When employing a monocular trial, we assume that the spontaneous IOP variation between an individual’s two eyes is identical, or at least very similar. By making this assumption, we can factor out the spontaneous variability based upon the IOP change in the untreated control eye and determine the selected agent’s true therapeutic effect in the treated eye. However, we now know that this is not a valid assumption.
One study looked at the frequency of asymmetric IOP fluctuations––both in healthy patients and those with glaucoma. The researchers found that spontaneous asymmetric fluctuations of IOP between fellow eyes occur commonly in normal subjects and glaucoma patients. The documented IOP fluctuations between consecutive clinical visits in glaucoma patients were too significant to confuse with the therapeutic effect of their glaucoma medications.2
Another study that looked at IOP variability found that, while there is a concordance in IOP variation in fellow eyes of patients with glaucoma, contralateral eye IOP may indeed vary in an asymmetric fashion.3
The monocular trial assumes that the spontaneous diurnal variation is similar both in the short term as well as the long term. That is, when testing medication efficacy, bringing the patient back to measure IOP at the same time of day should eliminate or at least reduce spontaneous IOP variability. Further, this spontaneous IOP variability should be very similar months and years later in the same patient.
However, research now clearly indicates that these assumptions are invalid.
In one study, researchers found that treated glaucoma patients did not manifest a repeatable diurnal IOP pattern from day to day.4 Even in healthy individuals, there is not a sustained and reproducible diurnal IOP pattern, and a single-day assessment of IOP incompletely characterizes the diurnal IOP pattern.5
The final assumption critical to the use of the monocular trial is that the therapeutic response in one eye will be similar to that observed in the fellow eye. However, it appears that this assumption also is incorrect.
One group of researchers tried to predict the response of glaucoma therapy in one eye based upon the response in the fellow eye and found that the change in IOP in one eye did not accurately predict the therapeutic response in the fellow eye.6
Another research team also examined the therapeutic response of fellow eyes in monocular trials to determine if the IOP reduction observed in the first eye correlated with that seen in the fellow eye. Similarly, they found that if a medication yielded a significant IOP-reducing effect in one eye, the fellow did not necessarily exhibit a similar response.7
To reduce the confounding factor of systemic absorption and crossover effects, the researchers also performed a sub-analysis on patients using latanoprost––which has little, if any, contralateral IOP effect due to rapid systemic metabolism––and still found a poor prediction in IOP effects between the two eyes.7
What Do We Do?
The aforementioned assumptions upon which monocular trials are based are largely unsupported. In fact, IOP variability in fellow eyes is asymmetric, and the diurnal IOP variation is inconstant. Further, therapeutic response in one eye is not indicative of the effect in the other eye.
For these reasons, perhaps we should consider abandoning the monocular trial altogether. We simply cannot be confident that a fellow eye can serve as a control.
By using monocular trials, we could erroneously determine that a patient is a non-responder when, in fact, the opposite is true. Additionally, if you performed monocular trials often and always treated the eye with the higher IOP first, you could incorrectly assume that a medication worked better than it really did simply by observing regression to the mean (extreme values tend to be less significant upon reassessment).
Monocular trials seem to provide little useful information and potentially could delay therapeutic glaucoma treatment. Long-term IOP control cannot be determined simply by comparing a single pre-treatment IOP with a single post-treatment IOP response in one eye.
Preferentially, when initiating glaucoma therapy for a patient, if both eyes need medication, start with bilateral treatment. Examine the IOP range over several visits prior to treatment, and then look at the IOP trend following treatment over a period of time. That is likely the only way to truly understand the efficacy of long-term IOP control in both eyes.
1. Shields MB. Textbook of Glaucoma. 4th ed. Baltimore: Williams & Wilkins; 1998.
2. Realini T, Barber L, Burton D. Frequency of asymmetric intraocular pressure fluctuations among patients with and without glaucoma. Ophthalmology. 2002 Jul;109(7):1367-71.
3. Dinn RB, Zimmerman MB, Shuba LM, et al. Concordance of diurnal intraocular pressure between fellow eyes in primary open-angle glaucoma. Ophthalmology. 2007 May;114(5):915-20.
4. Realini T, Weinreb RN, Wisniewski S. Short-term repeatability of diurnal intraocular pressure patterns in glaucomatous individuals. Ophthalmology. 2011 Jan;118(1):47-51.
5. Realini T, Weinreb RN, Wisniewski SR. Diurnal intraocular pressure patterns are not repeatable in the short term in healthy individuals. Ophthalmology. 2010 Sep;117(9):1700-4.
6. Chaudhary O, Adelman RA, Shields MB. Predicting response to glaucoma therapy in one eye based on response in the fellow eye: the monocular trial. Arch Ophthalmol. 2008 Sep;126(9):1216-20.
7. Realini T, Fechtner RD, Atreides SP, Gollance S. The uniocular drug trial and second-eye response to glaucoma medications. Ophthalmology. 2004 Mar;111(3):421-6.

