Her ocular history was remarkable for significant peripapillary atrophy in both eyes. The patient’s systemic history was remarkable for asthma, seasonal allergies and mild anemia.
Diagnostic Data
The patient’s best-corrected visual acuity was 20/50 O.D. and 20/25 O.S. Ishihara color vision testing was 3/14 in the right eye and 12/14 in the left eye. Pupils were equally round and reactive to light, with no afferent pupillary defect. Extraocular muscle movement was full.
Slit lamp examination revealed moderate nuclear sclerotic and cortical cataracts in both eyes. Intraocular pressure was 21mm Hg O.D. and 22mm Hg O.S. using Goldmann applanation tonometry. Dilated fundus exam showed healthy optic nerves, with cup-to-disc ratios of 0.70 x 0.60 O.D. and 0.70 x 0.70 O.S. Significant peripapillary pigmentary changes were noted greater in the left eye.
There was a subtle serous detachment of the neurosensory retina in the macula of the right eye extending to the nasal border of the optic nerve (figures 1A and 1B). Mild retinal exudate was also present in that region.
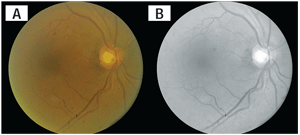
1A. A color fundus photograph of the
right eye. Note the shallow macular serous retinal detachment extending
to optic nerve with mild retinal exudation. 1B. A red-free fundus
photograph of the same eye. Note the enhanced visualization of the
macular serous detachment.
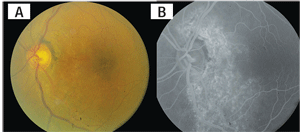
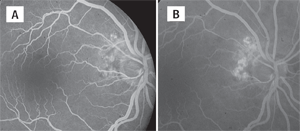
2A. A fundus photograph of the left eye
shows prominent peripapillary atrophy. The retinal pigment epithelial
changes around the optic nerve continue in the inferior temporal arcade
and foveal area. 2B. A fluorescein angiogram of the left eye at 3
minutes 25 seconds reveals significant window defects that highlight
the extent of the retinal pigmentary alterations.
In left eye, there were significant retinal pigment epithelial changes extending from around the optic nerve to the inferior temporal arcade and foveal areas (figures 2A and 2B).
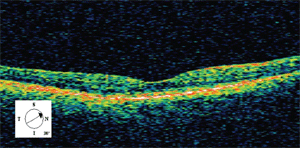
Optical coherence tomography showed a serous neurosensory retinal detachment of the macula in the right eye extending to the optic nerve (figures 3A and 3B). Retinal topography of the macula in the left eye revealed mild general thinning suggestive of retinal atrophy, corresponding to the area of retinal pigmentary alterations (figure 4).
Fluorescein angiography (FA) of the right eye disclosed an abnormal choroidal juxtapapillary vascular network as the source of the subretinal fluid. There were areas of early focal hyperfluorescence with mild late staining indicative of leakage of this choroidal vascular network (figures 5A and 5B). FA of the left eye revealed the extent of the retinal pigment mottling (figure 2B).
Diagnosis
Based on the clinical presentation, a serous neurosensory macular detachment associated with polypoidal choroidal vasculopathy (PCV) was diagnosed in the right eye. The retinal pigment changes and atrophy observed in the left eye were postulated to have been caused by a previous exudative neurosensory detachment associated with PCV. Although indocyanine green angiography (ICGA) would have enhanced visualization of the choroid, the retinologist believed it was not necessary, and that the FA findings, OCT and funduscopy were adequate to make the diagnosis of PCV.
Treatment and Follow-up
Focal laser photocoagulation was applied to the peripapillary polypoidal lesion.
At two months, the subretinal fluid was resolving. At four months, there was complete resolution of the subretinal fluid and BCVA improved to 20/30 O.D. No additional laser treatments were required.
The lesion remained inactive at sixteen months after laser treatment, and the final visual acuity was 20/30 in the right eye (figure 6).
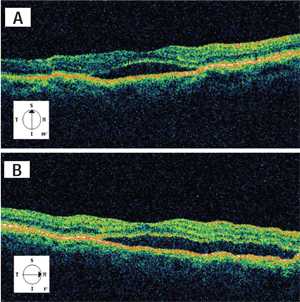
3A. OCT of the macula in right eye
demonstrates a neurosensory retinal detachment. 3B. This
temporal-to-nasal cross-section shows the serous retinal detachment
extending towards the optic nerve.
The patient was scheduled for a follow-up visit in one year.
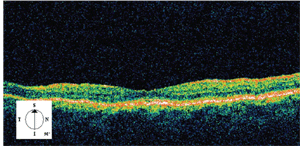
4. OCT of the macula in left eye reveals mild general retinal thinning.
Discussion
More than 20 years ago, a “peculiar hemorrhagic disorder of the macula involving serosanguinous detachments of the retinal pigment epithelium and neurosensory retina” was described by Yannuzzi at a macular society meeting in 1982.1 This condition has been given many names since, including “multiple recurrent retinal pigment epithelial detachments in black women,” “posterior uveal bleeding syndrome,” and “idiopathic polypoidal choroidal vasculopathy.” More recent reports have termed this clinical entity as polypoidal choroidal vasculopathy without “idiopathic” because it is now believed to be caused by a particular form of choroidal neovascularization (CNV) similar to—but also unique from—that of age-related macular degeneration.2
Polypoidal choroidal vasculopathy (PCV) is characterized by a network of branching inner choroidal vessels with terminal, polyp-like aneurismal dilations.1 Opthalmoscopically, they appear as multiple reddish-orange protrusions from the choroid into the subretinal space.2 These lesions are often associated with recurrent exudative and hemorrhagic detachments of the neurosensory retina and RPE.3,4 Vitreous and subretinal hemorrhage with relatively minimal fibrous scarring may also occur.3,4
Polypoidal CNV is considered to be a unique form of CNV with characteristics distinguishing it from that of AMD. In PCV eyes studied by Uyama and colleagues, neither occult nor classic CNV was observed.19 Also, there was no development of polypoidal CNV through the RPE into subretinal space. In addition, none of the eyes had serofibrinous exudation or fibrovascular membrane which are frequently seen with exudative AMD.19 It was postulated that the role of the RPE differs in the development of CNV between PCV and AMD.19
Demographics
Although PCV was initially described in black women, our increasing understanding and awareness of this clinical entity illustrates that this disease is prevalent in all races and both sexes.18,44,45 Studies suggest a significantly higher predilection among more heavily pigmented individuals including blacks, Asians and Hispanics when compared to whites in contrast to the incidence of AMD.19,23,44-48 The usual age of onset of polypoidal CNV ranges between 50 and 65 years with an average of 60.1 years.4,45 However, the age of diagnosis ranges between the 20s and the 80s.4,45 This is much younger and broader than seen with AMD. In addition, white patients typically present with the disease at an older age.46
The demographics and clinical features of PCV have been found to differ greatly among different ethnic groups.5,21 Yannuzzi and associates described a predominance of women affected with PCV by a ratio of 4.7:1, with a tendency for bilateral involvement and peripapillary location of polypoidal lesions in white patients.4,45 Studies in Asia have found a preponderance of men affected with the disease with commonly unilateral involvement occurring frequently in the macular region in these patients.5,19,21,47,49
5A. FA of the right eye at 20 seconds
highlights the abnormal peripapillary choroidal vascular network. 5B.
FA at 2 minutes and 30 seconds shows mild late staining and leakage in
the same region.
Etiology
6. OCT of the right eye at six-month follow-up reveals complete resolution of the serous fluid.
The pathogenesis of PCV is not clearly understood. Currently, there are no clear links between PCV and associated systemic disease. In the past, PCV was thought to be caused by a microvascular disease process such as hypertension and diabetes.6-8 However, the prevalence of systemic microvascular disease is equivalent between the general population and the subset with polypoidal CNV.2
Researchers have proposed a possible role of biochemical mediators of vasogenesis, including vascular endothelial growth factor (VEGF), that may be responsible for the neovascularization seen in PCV. Various reports have shown strong expression of VEGF in PCV specimens and also its upregulation in the aqueous humor in eyes with PCV.24,25 Recent studies have also located a complement factor H gene with strong association with PCV as a probable factor leading to its development.9
Interestingly, a common genetic link has also been found between PCV and AMD. Gotoh and associates located an ARMS2 (LOC387715) genetic variant in Japanese patients with strong associations with both exudative AMD and PCV.10
The abnormal vascular network in PCV is consistent histopathologically with the short posterior ciliary arteries and therefore thought to originate from its branches, which develop through defects in Bruch’s membrane.11 The exact location of this vascular network remains unclear. Clinicopathologic studies have reported these thin-walled bulbous structures as a sub-RPE, intra-Bruch’s fibrovascular membrane.13,14 Okubo and associates suggested that these lesions can be more accurately considered as a degenerated RPE-Bruch’s membrane-choriocapillaris complex and inner choroid dilated venules and arterioles, rather than an intra-Bruch’s fibrovascular membrane.16
OCT studies show that these lesions cause sharp protrusions of the RPE corresponding to those seen on indocyanine green angiography and clinical examination.15 These lesions are most commonly located in the peripapillary area, with the macula being the second most common, and can occur in the peripheral fundus as well.2 The lesions may also be single and isolated, or widespread and multiple.4
PCV is considered a bilateral disease since many patients who present unilaterally have been found to eventually develop lesions in the fellow eye.4 However, there are still several cases in which patients have been followed for more than 10 years and continue to show no evidence of PCV in the uninvolved eye.17
Imaging and Diagnosis
The most common clinical features associated with PCV are exudative and hemorrhagic detachments of the neurosensory retina and RPE.4 Studies in Japan reported 55% to 85% of eyes with hemorrhagic and exudative neurosensory and RPE detachments were found to have PCV.5 Serous macular detachments were also commonly accompanied by PCV.18
On FA, the PCV lesion often appears to have occult characteristics with early mottled hyper- and hypofluorescence.2,5 Because of this, macular PCV lesions are often misdiagnosed as AMD when visualized with FA alone.5 Indocyanine green angiography (ICGA) has been adopted as the best diagnostic tool for the visualization of polypoidal CNV because of its ability to highlight the choroidal vasculature.2
The PCV branching networks underneath the RPE are clearly visible with ICGA. In the early stage, the location of the networks become easily identifiable with the filling of the larger vessels of the PCV network preceding the retinal vessels.4 Subsequently, small focal hyperfluorescent “polyps” become apparent in areas of the network with terminal vessel aneurismal dilations, corresponding to the reddish-orange protrusions visible on biomicroscopy.2,4
Then, in the later stages, a reversal of the pattern of fluorescence is seen, with the center of the polypoidal lesion becoming hypofluorescent while the surrounding area exhibits hyperfluorescence.2,4 There is a vanishing of the dye, or “washout” of the lesion, observed in the very late stages in only non-leaking PCV lesions.4 Hyperfluorescence will continue to be observed throughout the late phases in leaking polypoidal lesions. The Japanese study group of Polypoidal Choroidal Vasculopathy has proposed criteria for the diagnosis of PCV largely based on ICGA and biomicroscopy.12 For instance, definite cases meet at least one of the following criteria: Protruded orange-red elevated lesions are observed by fundus examination; and/or characteristic polypoidal lesions are seen in ICGA findings.12
OCT has recently been proven to be useful in the diagnosis of PCV. It allows for easy identification of serous RPE or neurosensory detachments, in particular if the presentation is subtle. On OCT, polypoidal lesions appear as sharp dome-like elevations of the RPE with moderate inner reflectivity.15 In addition, a highly reflective line just below the mildly elevated and reflective RPE was often observed, consistent with the location of the vascular branching networks.15 Sato and associates called the dual reflective layers the “double-layer sign,” which they observed in 59% of eyes with PCV.20
Natural Course
The visual prognosis of PCV is more favorable than that of exudative AMD. Nevertheless, significant and permanent visual loss can occur with macular involvement of PCV. Uyama and associates followed 14 eyes of 12 patients for at least two years without any treatment.19 They found that 50% of patients had a favorable course with visual acuity greater than 20/30, while the remaining 50% suffered persistent macular disease and poor visual outcome due to chronic and recurrent bleeding and leakage, leading to RPE degeneration and atrophy.
Eyes with the highest risk for severe visual loss were those with a cluster of grape-like polypoidal dilations of the vessels, which were associated with marked bleeding and leakage. Eyes with solitary round aneurismal dilations had the most favorable course, as these lesions often regressed. Changes in the configuration of the terminal dilations were variable, and occurred as quickly as a few months to as slowly as a year. Although clinical manifestations of PCV and AMD appear similar, PCV often occurs with minimal fibrous proliferation and scarring.19 This factor, in addition to its slower progression, contributes to the better prognosis in comparison with AMD.4,21
Differential Diagnosis
The differential diagnosis of PCV includes the spectrum of disease processes that involve CNV, and exudative and hemorrhagic retinopathy. With many clinical similarities, it is not surprising that the most common misdiagnosis of PCV is AMD.2 Maruko and associates reported a prevalence rate of 54% for PCV in eyes of Japanese patients previously misdiagnosed with AMD.22 Ahuja and associates found that in eyes that manifested large hemorrhagic and exudative neurosensory retinal and RPE detachments greater than 2mm in diameter and in the absence of drusen consistent with PCV, all cases were confirmed to have PCV with ICGA.23
The most common misdiagnosis in this study was idiopathic central serous chorioretinopathy and AMD. Some patients with PCV present with exudation that is predominantly serous masquerading as central serous chorioretinopathy.4,18
Other differential diagnoses include retinal telangiectasias, retinal macroaneurysms, valsalva retinopathy, and Purtscher’s retinopathy when the appearance is primarily hemorrhagic.2 Other impersonators include inflammatory processes and conditions that predispose patients to the development of CNV, such as ocular histoplasmosis, angioid streaks, pathologic myopia, choroidal rupture, and choroidal tumors and hemangiomas.2
Treatment Options
An accurate diagnosis of PCV is essential for recommending appropriate treatment and management options.
A conservative approach to management can be taken when changes do not threaten the central macula.4 We know that about 50% of patients with PCV have good visual prognosis on observation alone, and that polypoidal lesions may undergo spontaneous regression.19
In cases in which persistent or progressive exudative changes threaten central vision, conventional laser photocoagulation to the leaking polypoidal lesion can be considered. Laser photocoagulation has been shown effective for the resolution of serous fluid and reabsorption of blood.26-28 Nishijima and associates showed that 60% of patients had complete resolution of the serous retinal detachment when laser photocoagulation was applied to ICGA-identified polypoidal feeder vessels.29 However, thermal laser should not be applied to subfoveal lesions due to its risk of damaging the overlying neurosensory retina with subsequent reduction in visual acuity.2
Photodynamic therapy (PDT) using verteporfin has been proven to be safe and effective for subfoveal polypoidal lesions.4,5,30-32 Quaranta and associates followed 30 eyes with subfoveal PCV lesions with exudative retinal detachment and hemorrhage over a period of 12 months.30 They found that with a mean treatment number of 2.5, there was complete resolution of the serous macular detachments in 83.3% of eyes and complete occlusion of the polypoidal dilations in 73.3%. Chan and associates studied 22 eyes treated with ICGA-guided PDT using verteporfin.32 Stable or improved vision was observed in 95% of eyes, absence of leakage in FA in 91%, and total regression of polyps in 95% with an average of 1.6 treatments.
Gomi and associates compared one-year results of PDT between Japanese patients with ARMD and those with PCV, and found significantly better visual and angiographic outcomes with PCV.33 Visual acuity improved by 25% in PCV vs. 6% in AMD, and decreased by 8% and 31% respectively. Leakage in FA stopped at one year in 86% of PCV and 61% of AMD eyes. Additional PDT treatments were performed in 67% of eyes with AMD and in 39% of eyes with PCV, with PCV eyes having a lower average number of treatments of 1.5 vs. 2.0 with AMD. However, recurrence or the development of new polyps was seen in 8.4% of PCV eyes one year after the first treatment.
Recurrent PCV, persistent branching networks, and repeated subretinal hemorrhage and leakage, unfortunately, have also been frequently observed by multiple investigators during long-term follow-up after treatment with PDT.34-36 Yamashiro and associates reported an estimated recurrence rate of up to 40% of polypoidal lesions at 21 months post-treatment.39 Subretinal and sub-RPE hemorrhage within one month of treatment with PDT have also been reported to be a common complication.32,33,37,38
Anti-VEGF therapy, which has been used in recent years in the treatment of CNV associated with AMD, is being explored as a possible treatment for polypoidal CNV. Ghajarnia and associates reported that the use of bevacizumab resulted in resolution of subretinal fluid and improvement in vision in a patient with refractory PCV previously treated with PDT and pegaptanib.43 Gomi and associates found that intravitreal bevacizumab caused a temporary decrease in exudation, but the choroidal vascular abnormalities still remained in ICGA in 10 of 11 eyes.40
The decreased efficacy of bevacizumab may be due to one of two reasons: limited penetration with the sub-RPE location of the polypoidal vascular abnormalities, or that VEGF may play a more limited role in the pathogenesis of PCV.5 Lai and associates had similar findings.42 Bevacizumab was effective in stabilizing visual acuity and reducing exudation but had limited efficacy in causing regression of polyps.
Also, patients with subsequent PDT were more likely to have regression of the polypoidal lesions in ICGA, suggesting a possible role for combination therapy.
Reche-Frutos and associates studied the effect of ranibizumab on PCV and demonstrated a beneficial short-term effect of monthly intravitreal injections for both the reduction of subretinal fluid and regression of polyps.41
Polypoidal choroidal vasculopathy is a distinct, though relatively new, clinical entity that should not be overlooked in the case of any patient presenting with serous, exudative and hemorrhagic retinopathy, especially in the absence of drusen. Our more recent recognition of this and our growing understanding of its spectrum of clinical characteristics have made this disease much more common than previously appreciated.11,18,44,45,46,47
PCV has unique clinical and angiographic features that differentiate it from other causes and forms of choroidal neovascularization. Although indocyanine green angiography has shown to be most effective for visualizing PCV lesions, clinical presentation, FA and OCT have also proven useful in the diagnosis.
Photodynamic therapy with verteporfin, laser photocoagulation, and anti-VEGF therapy have all been found beneficial in minimizing visual deterioration. Although our knowledge of PCV continues to expand, there is much about its pathogenesis that remains unclear. A more detailed pathogenesis will be the key in establishing more effective treatment options with future clinical trials.
Dr. Chen is a clinical faculty member at the Pennsylvania College of Optometry at Salus University. Dr. Pelino is an assistant professor at the Pennsylvania College of Optometry and director of the optometric retina service at The Eye Institute. He also co-authors Review’s “Review of Systems” column.
1. Yannuzzi LA. Idiopathic polypoidal choroidal vasculopathy. Presented February 5, 1982 at the Macula Society Meeting, Miami, Florida.
2. McCleary CD, Guier CP, Dunbar MT. Polypoidal choroidal vasculopathy. Optometry. 2004 Dec; 75(12):756-70.
3. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002 May; 133(5):639-48.
4. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004 Jan-Feb; 49(1):25-37.
5. Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008 May; 19(3):208-12.
6. Ciardella AP, Donsoff IM, Yannuzzi LA. Polypoidal choroidal vasculopathy. Ophthalmol Clin North Am. 2002 Dec; 15(4):537-54.
7. Lip PL, Hope-Ross MW, Gibson JM. Idiopathic polypoidal choroidal vasculopathy: a disease with diverse clinical spectrum and systemic associations. Eye. 2000 Oct; 14 Pt 5:695-700.
8. Ross R, Gitter K, Cohen G, et al. Idiopathic polypoidal choroidal vasculopathy associated with retinal arterial macroaneurysm and hypertensive retinopathy. Retina. 1996; 16:105-11.
9. Kondo N, Honda S, Kuno S, Negi A. Coding variant 162V in the complement factor H gene is strongly associated with polypoidal choroidal vasculopathy. Ophthalmology 2009 Feb; 116:304-310.
10. Gotoh N, Nakanishi H, Hayashi H, et al. ARMS2 (LOC387715) variants in Japanese Patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009 Jun;147(6):1037-41, 1041.e1-2.
11. Moorthy RS, Lyon AT, Rabb MF, et al. Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology 1998 Aug; 105:1380-5.
12. Japanese Study Group of Polypoidal Choroidal Vasculopathy. Criteria for diagnosis of polypoidal choroidal vasculopathy. J Jpn Opthalmol Soc. 2005 Jul;109(7):417-27.
13. Spraul CW, Grossniklaus HE, Lang GK. Idiopathische polypose choroidale vaskulopathie (IPCV). Klin Monatsbl Augenkeilkdd 1997; 210:405-6.
14. Lafaut BA, Aisenbrey S, Van den Broecke C, et al. Polypoidal choroidal vasculopathy pattern in age-related macular degeneration. A clinicopathologic correlation. Retina 2000; 20:650-4.
15. Kamaeda T, Tsujikawa A, Otani A, et al. Polypoidal choroidal vasculopathy examined with en face optical coherence tomography. Clin Experiment Ophthalmol. 2007 Sep-Oct;35(7):596-601.
16. Okubo A, Sameshima M, Uemura A, et al. Clinicopathologic correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Opthalmol. 2002; 86:1093-1098.
17. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy. Retina. 1990; 10:1.
18. Yannuzzi LA, Freund KB, Goldbaum M, et al. Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology. 2000 Apr;107(4):767-77.
19. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002 May; 133(5):639-48.
20. Sato T, Kishi S, Watanabe G, et al. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007 27; 589-594.
21. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003 Oct; 121(10):1392-6.
22. Marko I, Iida T, Saito M, et al. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Opthalmol. 2007 Jul;144(1):15-22.
23. Ahuja RM, Stanga PE, Vingerling JR, et al. Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelial detachments. Br J Ophthalmol. 2000 May;84(5):479-84.
24. Tong JP, Chan WM, Liu DT, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol 2006; 141:456-462.
25. Matsuoka M, Ogata N, Otsuji T, et al. Expression of pigment epithelium growth factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol 2004; 88:809-915.
26. Gomez-Ulla F, Gonzales F, Torreiro MG. Diode laser photocoagulation in idiopathic polypoidal choroidal vasculopathy. Retina 1998; 18:481-483.
27. Yuzawa M, Mori R, Haruyama M., A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003 Jul-Aug; 47(4):379-84.
28. Guyer DR, Yannuzzi LA, Ladas I. Indocyanine-green guided laser photocoagulation of focal spots at the edge of plaques of choroidal neovascularization. Arch Ophthalmol 1996; 114:693-7.
29. Nishijima K, Takahashi M, Akita J, et al. Laser photocoagulation of indocyanine green angiographically identified feeder vessels to idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2004 Apr; 137(4):770-3.
30. Mauget-Faÿsse M, Quaranta-El Maftouhi M, De La Marnièrre E, Leys A. Photodynamic therapy with verteporfin in the treatment of exudative idiopathic polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2006 Sep-Oct; 16(5):695-704
31. Otani A, Sasahara M, Yodoi Y, et al. Indocyanine green angiography: guided photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007 Jul; 144(1):7-14.
32. Chan WM, Lam DS, Lai TY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy. Ophthalmology 2004; 111:1576-1584.
33. Gomi F, Ohji M, Sayanagi K, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008 Jan; 115(1):141-6.
34. Akaza E, Mori R, Yuzawa M. Long-term results of photodynamic therapy of polypoidal choroidal vasculopathy. Retina. 2008 May;28(5):717-22.
35. Silva RM, Figueira J, Cachulo ML, et al. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol. 2005; 243:973-979.
36. Sayanagi K, Gomi F, Sawa M, et al. Long-term follow-up of polypoidal choroidal vasculopathy after photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol. 2007; 245(10):1569-71.
37. Lee SC, Seong YS, Kim SS, et al. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004; 218:193-201.
38. Hirami Y, Tsujikawa A, Kameda T, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007; 27:335-341.
39. Yamashiro K, Tsujikawa A, Nishida A, et al. Recurrence of polypoidal choroidal vasculopathy after photodynamic therapy. Jpn J Ophthalmol. 2008; 52(6):457-62.
40. Gomi F, Sawa M, Sakaguchi H, et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92(1):70-3.
41. Reche-Frutos J, Calvo-Gonzales C, Donate-Lopez J, et al. Short-term effect of ranibizumab for polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2008; 18(4):645-8.
42. Lai TY, Chan WM, Liu DT, et al. Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008 May;92(5):661-6.
43. Ghajarnia M, Kurup S, Eller A. The therapeutic effects of intravitral bevacizumab in a patient with recalcitrant idiopathic polypoidal choroidal vasculopathy. Semin Ophthalmol 2007; 22:127-31.
44. Yannuzzi LA, Ciardella A, Spaide RF, et al. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 1997; 115:478-485.
45. Yannuzzi LA, Wong DWK, Storzolini BS, et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol 1999; 117:1503-1510.
46. Lafaut BA, Leys AM, Snyers B, et al. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000 Sep;238(9):752-9.
47. Uyama M, Matsubara T, Fukushima I, et al. Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol 1999; 117:1035-1042.
48. Stern RM, Zakov N, Zegarra H, et al. Multiple recurrent serosanguinous retinal pigment epithelial detachments in black women. Am J Ophthalmol 1985; 100:560-569.
49. Kwok KAH, Lai TYY, Chan CWN, et al. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol 2002; 86:892-897.

