Refractive surgery comanagement continues to be an integral part of optometric practice. As with any technology, advances are occurring rapidly. As primary eye care providers, we can offer our patients accurate information so they can enjoy clear vision for their lifetimes.
Here, as part of Review of Optometrys 10th Annual Comanagement Report, well look at the latest advances in refractive surgery, their advantages and disadvantages and the appropriate candidates for each procedure.
 |
| Optometrists need to stay informed about advances in refractive surgery so we can be the primary resource of information and referrals for our patients. |
Wavefront-Guided LASIK
Although wavefront-guided LASIK has not consistently delivered the super vision1 we had hoped for, this technology allows us to understand vision better than ever before. Wavefront, which applies the principles of adaptive optics, offers us ways of assessing lower- and higher-order aberrations of the eye.
Wavefront technology will likely be incorporated into all forms of vision correction eventually. Spectacle and contact lens manufacturers already offer products that measure and correct for higher-order aberrations.
But how much information do we really need? Zernike coefficients, mathematical ways of assessing lower- and higher-order aberrations, can measure up to 11th-order aberrations. R. Krueger and colleagues, however, have found that aberrations above the sixth order are inconsequential.2 Coefficients above the sixth order actually introduce visual noise from tear film debris and instability, vitreous floaters and corneal disruptions. These factors will adversely affect the outcome if used to decide the patients correction.
Furthermore, the root mean square deviation, or RMS values, can vary between blinks, mostly due to tear film dynamics. We use RMS values to quantify aberrations. A lower RMS value in the human eye means less higher order aberrations. And, controlling accommodation during testing is difficult.
Additionally, those researchers who are still trying to find ways to achieve super vision of 20/8 will need to address whether the human visual cortex is capable of such resolution.
So, who should undergo wavefront-guided LASIK? A patient whose RMS value is greater than 0.3m has visually significant higher-order aberrations and would benefit from wavefront. But even more basic guidelines can help us decide which patients will benefit most from wavefront-guided LASIK.
Pupil size remains extremely important when deciding if a patient will benefit from wavefront laser technology. Generally, a patient with a low spherical Rx (with or without a small amount of astigmatism), small pupils (<6.0mm) and low RMS values (<0.30m) would most likely do fine with a traditional ablation. Most patients with higher refractive error, with or without large pupils, would probably benefit from wavefront vs. traditional LASIK, no matter their RMS value. And most patients with large pupils, whatever their refractive error, would probably benefit from wavefront vs. traditional LASIK, regardless of their RMS value.
Even if wavefront does not deliver the super vision that we had originally hoped it would, it is still less likely than traditional LASIK to induce new aberrations. This alone may be the most valuable quality of wavefront-guided LASIK today.
| Table 1. Wavefront-Guided Lasers There are currently three FDA-approved wavefront-guided lasers:
|
We now have two ways to create the LASIK flap: the traditional method using the keratome or the IntraLase femtosecond (FS) laser. But is one way better than the other?
IntraLase FS creates the flap through photodisruption and hydrodissection of the stromal tissue. Specifically, it creates thousands of 2m to 3m capitation bubbles within the cornea. This technically destroys tissue and creates a space in which the flap can be separated from the bed.
In one study, researchers compared patients who underwent LASIK with IntraLase to those who underwent LASIK with two popular microkeratomes and found that the IntraLase demonstrated more predictable flap thickness, better astigmatic neutrality and decreased epithelial injury.3 Another study also found that the IntraLase laser can predictably create flap diameters, hinge location and flap thickness while eliminating the risk for cap perforations.4
While IntraLase does not carry the same risks as the keratome, it still carries risks. The energy and fluid from the IntraLase yield more corneal edema, and patients typically require stronger steroid drops for a longer period than patients whose flap was created with the keratome. Flap creation with the IntraLase can take one to two minutes (depending on the skill of the surgeon) vs. less than 30 seconds with the microkeratome. During this time the suction ring is in place, which can lead to increased pain and discomfort.
Because the bubbles are not contiguous, the IntraLase flap tends to stick to the corneal bed due to micro-adhesions of collagen fibrils. These adhesions are broken when the flap is lifted and can create a bed that is not perfectly smooth.
 |
| The IntraLase femtosecond laser creates a flap through photodisruption and hydrodissection of the stromal tissue. |
Both IntraLase and microkeratome procedures are safe and effective, with no statistically significant difference in visual outcomes between the two procedures.6 Flap complications such as striae/microfolds, buttonhole flaps, epithelial ingrowth and infection occur at approximately the same rate with either procedure.6 Originally we expected that IntraLase would create a thinner flap than the keratome but a newer keratome, the Hansatome Excellus with the Zero Compression Head, creates ultra-thin flaps. IntraLase does create a flap with a more uniform thickness from edge to edge.
Eventually, the decision of which procedure the patient should undergo should be made on a case-by-case basis between the patient and his or her surgeon.
As femtosecond technology improves, shorter surgical times will likely reduce the edema and post-op recovery time. Reducing the amount of time our patients are using steroids will certainly reduce the incidence of steroid-related side effects. Additionally, studies are under way to determine if the femtosecond laser can be used to photoablate tissue intrastromally to correct vision without a flap at all. IntraLase can also be used to create the lamellar channels for Intacs and for therapeutic anterior lamellar keratoplasty.
Phakic IOLs
The FDA approved the first phakic IOL in September 2004. The Verisyse IOL, manufacturered by Ophtec and distributed by Advanced Medical Optics, is a PMMA implant that clips onto the mid-peripheral iris. The Verisyse lens can be implanted in patients who are at least 21 years old, have 5.00D to 20.00D of myopia, and up to 2.50D of astigmatism. Pupil size of 6.0mm or smaller is recommended.
Clinical studies have shown several positive advantages to the Verisyse.7 First, contrast sensitivity is preserved and often improved following the procedure compared with LASIK.8 Also, the rate of retinal detachment was not increased following phakic IOL surgery compared to lensectomy procedures.9,10 And, the preservation of the crystalline lens allowed for continued accommodation after treatment.9 (For more on visual results and complications, see tables 2 and 3.)
Table 2. Visual Outcomes of Verisyse Phakic IOL7
|
Patients anterior chamber depth and endothelial cell count will determine whether they are good candidates for this procedure. AMO has reported a loss of endothelial cells in patients who have undergone the procedure of 1.8% per year.11 At this rate, a patient will lose 50% of his or her endothelial cells after 25 years. We do not know all the consequences of this yet, but we know that if too many endothelial cells are lost, the patient will require a corneal transplant.
|
Table 3. Complications of the Verisyse Phakic IOL |
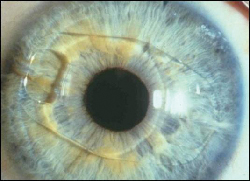 |
| Appearance of the Verisyse phakic intraocular lens in the eye after implantation. |
LASEK/PRK
We may think of photorefractive keratectomy (PRK) as a procedure of the past, but it still has a very important place in any refractive surgery practice. It may be the only choice for patients whose corneas are not thick enough to allow for a full-thickness flap and removal of tissue to attempt a plano target.
For patients with higher prescriptions (8.00D and above), some surgeons prefer laser epithelial keratomileusis (LASEK), even on patients who have enough corneal tissue to have LASIK because LASEK eliminates any risk of microfolds and may reduce the risk of corneal ectasia.
In LASEK, the surgeon scores the epithelium with a trephine, softens it with alcohol or methyl cellulose, then folds the epithelium back in one piece. Next, the surgeon photoablates the cornea, then folds the epithelium back on the cornea.
 |
| In LASEK, a trephine is placed over the cornea and filled with diluted alcohol. |
Surface scarring and haze have been a concern when performing PRK and LASEK. The incorporation of mitomycin C intraoperatively has reduced the incidence of scarring and haze following PRK/LASEK.12 Mitomycin C is an antibiotic/chemotherapeutic agent that inhibits DNA synthesis. The surgeon places a sponge soaked in mitomycin C on the cornea for about 60 seconds after the laser is performed. Patients should be informed that use of intraoperative mitomycin C may delay re-epithelialization.
Clear Lens Extraction
While clear lens extraction is possible and would work well for most patients with any refractive error, the risks may outweigh the benefit. Indeed, it may offer highly myopic patients distance acuity results that are superior to those of LASIK. However, the risks of intraocular surgery in an otherwise healthy and non-cataractous eyenamely hemorrhage, retinal detachment and endophthalmitisraises the question of whether it is ethical to perform a risky elective procedure.
Patients who may benefit the most from lens extraction are those older than age 50 and who already have some cataract with reduced accommodation. Theres decreased risk for detachment once the vitreous is forward. However, the group at substantial risk is patients younger than age 40, especially those who have a long eye (thin retina and sclera). Even with todays advancements in multifocal and accommodating IOLs, patients under 40 will lose some, if not all, of their accommodation. Additionally, some patients may need LASIK after implantation to correct residual refractive errors.
CK, a thermal, non-laser refractive procedure, was originally approved in 2002 for the correction of +0.75D to +3.25D of hyperopia with up to 0.75D of astigmatism. Clinical trials found the procedure to be safe and effective.13
In February 2004, CK was approved for presbyopia. Specifically, the FDA panel recommended that CK be approved for the temporary induction from 1.00D to 2.00D of myopia to improve near vision in the non-dominant eye of presbyopic emmetropes or hyperopes, in those with a successful history of monovision or a documented contact lens monovision trial. The panel limited the range of intended effect to 1.00D to 2.25D, or treatment with 16 or 24 spots. Some 86% of patients in this range achieved both uncorrected distance vision of 20/20 or better and near vision of J3 or better.12
Using a 450m stainless steel probe that delivers 350 kHz radio frequency energy, the surgeon places a series of circumferential spot applications to the mid-peripheral cornea. This causes the corneal collagen to contract like a belt, resulting in steepening of the central cornea. The effect of CK is determined by the number of spot applications, the number of rings of applications and the diameter of application rings.
 |
| CK uses radio frequency energy to place a series of spot applications on the mid-peripheral cornea. |
Treatment usually lasts for one to four years. Patients can be retreated but outcomes for retreatments are not yet published.
Refractive technology will continue to evolve and improve. Likewise, we need to continue to lead the field by promoting and supporting this technology. Most importantly, as optometrists, we need to stay informed about advances so we can continue to be the primary resource of information and referrals for our patients.
Dr. Bozich is in private practice in Los Altos, Calif. She has served as a staff optometrist for the Palo Alto Veterans Administration Department of Optometry and as a clinical faculty member at the University of California-Berkeley School of Optometry.
1. Catania LJ. The Refractive Evolution: Is 20/8 possible? Rev Optom 2002 Oct 15;139(10):52-62.
2. Krueger R, Applegate R, MacRae S, eds. Wavefront Customized Visual Correction: The Quest for Supervision. Thorofare, NJ: Slack Inc. 2004.
3. Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg 2004 Apr;30(4):804-11.
4. Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004 Jan;30(1):26-32.
5. Will BR. Track-related iridocyclitis and scleritis associated with the use of IntraLase for LASIK. Paper presented at: The ASCRS/ASOA Symposium on Cataract, IOL, and refractive Surgery; May 4, 2004, San Diego, CA, USA.
6. Durrie DS. Wavefront outcomes of IntraLase vs. Hansatome LASIK presented at : The 5th International Congress on Wavefront Sensing and Optimized Refractive Correction; February 22, 2004; Whistler, Ontario.
7. Ferguson SJ. Comanagement guide to phakic IOLs. Rev Optom 2004 Dec 15;141(12):42-4.
8. El Danasoury MA, El Maghraby A, Gamali TO. Comparison of iris-fixed Artisan lens implantation with excimer laser in situ keratomileusis in correcting myopia between -9.00 and -19.50 diopters: a randomized study. Ophthalmology 2002 May;109(5):955-64.
9. Pop M, Payette Y. Refractive lens exchange versus iris-claw Artisan phakic intraocular lens for hyperopia. J Refract Surg. 2004 Jan-Feb;20(1):20-4.
10. Jacobi FK, Hessemer V. Pseudophakic retinal detachment in high axial myopia. J Cataract Refract Surg 1997 Sep;23(7):1095-102.
11. FDA Clinical trial data. Published by AMO, Inc., Santa Ana, CA, 2004.
12. Siddique S. Paper presented at: The ASCRS/ASOA Symposium on Cataract, IOL, and Refractive Surgery; May 4, 2004, San Diego, CA, USA.
13. McDonald MB, Hersh PS, Manche EE, et al. Conductive keratoplasty for the correction of low to moderate hyperopia: U.S. clinical trial 1-year results on 355 eyes. Ophthalmology 2002 Nov;109(11):1978-90.

