Despite how commonplace cataract surgery has become, postoperative challenges may still occur, particularly when pre-existing retinal pathology is involved.
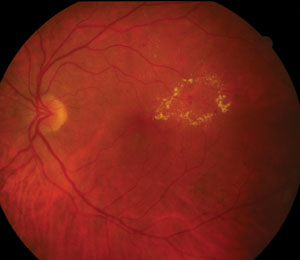 | |
| This patient has non-proliferative diabetic retinopathy with clinically significant macular edema. |
Multiple retinal conditions may impact cataract surgery outcomes, including disorders such as:
- Diabetic macular edema (DME)
- Retinal vein occlusion (RVO)
- Uveitis
- Epiretinal membrane formation
In addition, peripheral retinal lesions may also have bearing on complications. Peripheral findings that may be relevant to postoperative outcomes include lattice degeneration, retinal breaks, operculated holes, cystic retinal tufts and the whole family of pathologies related to vitreous traction.
Today, imaging tools allow a visual assessment of the posterior pole to a greater degree than ever before. Using this technology, we’ve been able to increase our understanding of the possible relationship between cataract surgery and some retinal pathologies.
This article will outline the more common conditions associated with a higher risk of postoperative complications of cataract surgery.
Epiretinal Membranes
Epiretinal membranes are common in potential cataract surgery patients.1 The Blue Mountains Eye Study shows the prevalence of an epiretinal membrane varies—estmating that 7% of patients older than 49 years of age will have an epiretinal membrane, 31% of which are bilateral.1 The incidence increases with age, as follows:1
- younger than 60 ..........1.7%
- between 60 and 69 ..........7.2%
- between 70 and 79 ..........11.6%
- 80 and older ..........9.3%
Some membranes are easily seen clinically whereas others are not. Spectral-domain OCT (SD-OCT) has allowed clinicians to visualize the anterior retina and the vitreoretinal interface with much greater detail than fundus photography and, in some instances, better than the clinical exam.
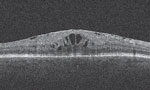 | |
| OCT reveals epiretinal membrane with cystoid macular edema. |
Cystoid macular edema (CME) is one complication of epiretinal membrane.2 It is important to identify macular edema when considering a patient for cataract surgery. Because the risk of post-cataract CME is increased in patients with an epiretinal membrane, any macular edema should be treated to establish some level of stability prior to clearing a patient for surgery.2 Although treatment of macular edema secondary to epiretinal membrane may not improve visual symptoms, it may reduce the risk of postoperative exacerbation.2
Retinal Vein Occlusions
Retinal vein occlusion (RVO) is the second most common retinal vasculature disease after diabetic retinopathy.3 When considering cataract surgery in a patient with a history of RVO, be sure the patient is completely free of retinal ischemia and check whether any macular edema is present. Fluorescein angiography (FA) is the standard diagnostic test for identifying capillary drop out and retinal ischemia. It should be performed on patients with an RVO, especially if the RVO is associated with central vision loss, disc edema, multiple hemorrhages, cotton-wool spots or macular edema.
Using SD-OCT, clinicians can evaluate the degree of macular edema through retinal contour maps, follow the response of treatment and adjust accordingly.
The goal of treatment is to suppress the edema; however, many patients with a retinal vein occlusion will require months of treatment. Intravitreal agents Lucentis (ranibizumab, Genentech), Eylea (aflibercept, Regeneron) and Ozurdex (dexamethasone, Allergan) are currently FDA approved for the treatment of macular edema secondary to a retinal vein occlusion. Avastin (bevacizumab, Genentech), although not FDA approved for use in the eye, is still a common first-line agent.
When considering cataract surgery in a patient with a history of a retinal vein occulusion, it is best to wait until the macular edema has resolved and remains stable for two to three months without intervention, unless the cataract precludes visualization of the posterior pole. Investigators have demonstrated that the risk of postsurgical cystoid macular edema in a patient with a history of a RVO is up to 30 times higher, and the risk persisted even in eyes without preoperative macular edema.2
Age-related Macular Degeneration
AMD remains the leading cause of legal blindness in patients older than 65, despite efforts to identify these individuals early in the course of the disease.4 Cataracts are the leading cause of reversible blindness worldwide and, ultimately, removal of a cataract will improve visual function whether or not the patient has AMD.4
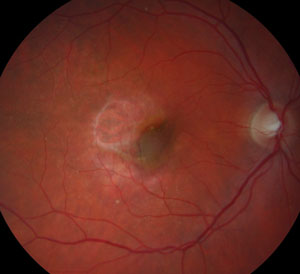 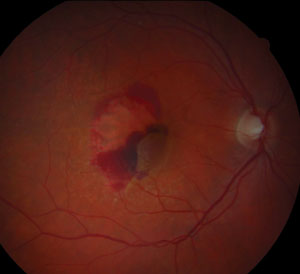 |
| At left, a fundus image of wet AMD post-injections and pre-cataract surgery. At right, wet AMD eight weeks post-cataract surgery with recurrent activity. |
The ongoing debate is whether uncomplicated cataract surgery can contribute to AMD progression. Some studies demonstrate that cataract surgery can improve visual function in the AMD patient in the short-term, but others associate cataract surgery as a risk factor for wet AMD in the long-term.5-7 As a result of conflicting studies and the inability to predict which patients are at high risk, practitioners are left to make a clinical judgment based on the limited data available.
The major protective filter to near ultraviolet radiation (300nm to 400nm) is provided by the crystalline lens, which becomes cloudy with cataract formation. Investigators theorize that this protects the retina against blue light in the visible spectrum and that a cataract may actually be somewhat protective against the development of AMD, making removal of the cataract a potential risk factor for AMD development.8 One study evaluated progression of AMD after unilateral cataract surgery in patients with bilateral symmetric findings of AMD. Exudative AMD developed in 19% of eyes that underwent cataract surgery compared with 4% of unoperated eyes.9 For this reason, investigators suggest reserving cataract surgery in AMD patients for those with reduced visual function. They also suggest close follow up of such eyes.6 A conflicting report concluded that cataract surgery did not increase the risk of developing exudative AMD.4
In contrast to potential exacerbation of AMD, we can look at the other side of the discussion and consider a gain in visual function by removing the cataract. Several studies conclude that by removing the cataract in an AMD patient, both visual function and quality of life were significantly improved.5,6
When considering cataract surgery in a patient with AMD, begin with an assessment of visual function objectively (e.g., snellen acuity, potential acuity, amsler grid testing, dark adaptation) as well as subjectively (e.g., night glare symptoms, light/dark adaptation complaints, or metamorphopsia). Then determine if the cataract itself is contributing to impaired visual function enough to consider the assessed risk of AMD progression.
Regarding patients with dry AMD, use an OCT to evaluate the integrity of the RPE, the size of drusen or pigment epithelial detachment present, and to rule out any cystoid macular edema or subretinal fluid that may not be clinically visible. Counsel patients with high-risk characteristics—such as soft, confluent drusen or the presence of a pigment epithelial detachment—on the increased risk of progression and follow them closely for the first 12 months postoperatively.
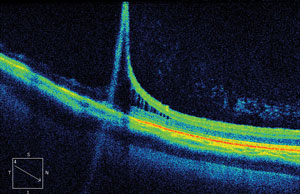  | |
| OCT scans allow practitioners to see changes in the peripheral retina, such as the retinoschisis in the top image. Below, a fundus image of bullous retinoschisis. |
When considering cataract surgery in a patient with prior wet AMD, you may suspect the risk of progression exceeds the risk of non-exudative AMD. However, the literature shows otherwise; it does not necessarily imply a causal link, and the timing of cataract removal may be of even greater consequence when dealing with patients who have a history of exudative AMD.10 Patients with exudative AMD are treated with anti-VEGF compounds monthly for an initial three months, and the retreatment is based on a PRN dosing vs. a treat-and-extend protocol. Although no concrete recommendation for the timing of cataract surgery exists, in my clinical experience it is commonplace to defer cataract surgery until the patient has not required intervention for a 90-day period. As with dry AMD, patients should be followed more closely during the postoperative period compared with a person without AMD. You can follow them using serial OCT images, but, in the event they develop CME or subretinal fluid, promptly provide a referral to a retina specialist.
With AMD, recommendations for cataract removal remain unclear, and oftentimes it becomes a personal decision made by the patient after you have discussed with them the risk factors associated with surgery and progression of AMD. Some features of exudative AMD—including an occult choroidal neovascularization (CNV), which is an under-recognized feature of AMD—may have few clinical findings. These findings include hemorrhage, edema or lipid seen with ophthalmoscopy, especially through a moderate to dense cataract. For these patients, SD-OCT is particularly helpful to identify cystoid macular edema or subretinal fluid that would be consistent with wet AMD, and treatment would be advised prior to removal of a cataract. Data from the AREDS study concluded there is a five-year progression risk of 12% to 50% in eyes with intermediate AMD, depending on pigment changes in addition to bilateral large-sized drusen.11 In addition, efforts are currently being made to determine high-risk patients, based on SD-OCT biomarkers.
Diabetic Macular Edema
More than 300 million people worldwide are affected by diabetes and related complications, including diabetic retinopathy and DME, which are the leading cause of visual impairment in working-aged Americans.12 Diabetes also increases the probability of developing a cataract and may contribute to an increased risk of reduced visual outcomes after cataract surgery.12,13 The preoperative work-up of a patient with diabetic retinopathy, DME or both should include careful examination of the posterior segment with or without fluorescein angiography. Consider SD-OCT imaging for all patients with reduced acuity not consistent with the degree of lens opacity. It should also be applied for all diabetic patients with microaneurysms or clinically significant macular edema, based on the established criteria defined by the ETDRS. This criteria includes:
- Retinal thickening at or within 500µm of the foveal center.
- Hard exudate at or within 500µm of the center of the fovea with adjacent retinal thickening.
- A zone of retinal thickening one disc area in size within one disc area of the center of the fovea.
One study demonstrated the incidence of developing center-involved macular edema in patients with no DME prior to cataract surgery was 0% at 16 weeks postoperatively. Eyes with a history of diabetic macular edema had different incidences of center-involved macular edema post-cataract surgery, depending on history of treatment. In patients with no history of treatment for DME, the incidence was 4%, whereas patients with a history of DME treatment had an incidence of 21% of developing center-involved edema.14
Consider SD-OCT imaging in all patients with diabetic retinopathy, and certainly those with macular edema, prior to cataract surgery to determine risk of complications.14 If center-involved edema is detected, refer the patient to a retina specialist. Postoperative care for the diabetic patient may require additional visits and, for those with a history of DME, serial SD-OCT images.
Peripheral Retinal Degenerations
Lattice degeneration is present in 7% to 8% of the general population, and of those cases up to 45% are bilateral.15 Lattice degeneration is associated with an increased risk of a retinal detachment—the incidence of which is about 1% in a person’s lifetime.16 When a patient with lattice degeneration is considered for cataract surgery, one management option includes prophylactic treatment. Given the low incidence of retinal detachment, it is unnecessary to treat all patients with lattice degeneration prophylactically. Atrophic holes associated with lattice degeneration increases the risk of retinal detachment to approximately 2% if asymptomatic prophylactic treatment has not been recommended.17 However, a consideration for treatment is made if the patient has symptoms of vitreous traction (e.g., flashing lights), the fellow eye has a history of retinal detachment or if the patient has a positive family history of a retinal detachment.
Cystic retinal tufts are chalky white in color, round or oval in shape and primarily composed of glial tissue. Their incidence in autopsy cases is 5%, with 20% of those presenting bilaterally.18 Despite being associated with 6.5% to 10% of non-traumatic retinal detachments, the risk of a retinal detachment in cystic retinal tuft patients is quite low and estimated to be only 0.28%.18 Prophylactic treatment of cystic retinal tufts is not necessary in cataract patients.
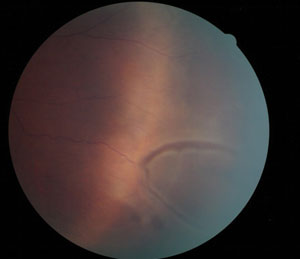 | |
| Horseshoe tears, such as this one, may progress to a retinal detachment. |
Symptomatic retinal breaks are associated with photopsia or an increase in floater symptoms. In a patient with a symptomatic posterior vitreous detachment (PVD), the risk of a retinal break is 10% to 15% and, if vitreous hemorrhage is present, the risk increases to 70%.19,20 Acute symptomatic horseshoe tears may progress to a retinal detachment in 30% to 50% of cases, and prophylactic treatment with laser photocoagulation or cryotherapy can reduce the risk of progression down to 5%.21,22 In contrast to horseshoe tears, symptomatic operculated breaks do not progress to a retinal detachment unless residual vitreous traction is seen on adjacent retinal tissue. Although this can be difficult to assess clinically, examination with scleral depression is useful. Treatment for operculated tears in the absence of intact vitreous traction is not advised prophylactically.
The peripheral retina is typically examined with an indirect ophthalmoscope as part of the evaluation prior to cataract surgery. Scleral depression may be part of such examination. While challenging to perform in some cases, scleral depression may provide additional information in that retinal findings may be viewed dynamically.
When evaluating a cataract patient who has peripheral retinal degeneration or retinal breaks, symptomatic or not, it is always a good idea to get surgical clearance from a retinal specialist.
Advances in imaging technology have also created ways to assess the peripheral retina before and after cataract surgery. Widefield photography and SD-OCT imaging allow a practitioner to identify, document, and follow retinal pathology.
The OCT image on page 60 documents the presence of a peripheral retinoschisis in a phakic patient. The character of the retinal layer separation is clear, and it will be helpful to know this if the patient is found to have any change after cataract surgery.
While the peripheral retina may be studied with these imaging methods, other areas of the posterior pole, especially the macula, may be evaluated as well. OCT scanning in particular has greatly expanded our understanding of the macula in its relation to disease. This has allowed for our increased ability to evaluate the macula in patients before and after cataract surgery.
Fortunately, in most instances patients who undergo cataract surgery have satisfactory results. While it is true that eye care practitioners need to be mindful of retinal conditions that may complicate cataract surgery, it is also worth noting the solid benefits that most achieve. With this in mind, ODs can move forward with the tools and knowledge that best serve those who need cataract surgery—even retina patients.
Dr. Haynie is the executive clinical director at Retina & Macula Specialists, with office locations in Tacoma, Renton, Olympia and Kennewick, Wash. He is a member of Thrombogenics’ advisory board.
1. Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevelence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997 June;104(6):1033-40.2. Henderson B, Kim J, Ament C, et al. Clinical pseudophakic cystoid macular edema-Risk factors for development and duration after treatment. J Cataract Refractive Surgery. 2007;33:1550-8.
3. Farah SE. The impact of cataract surgery on preexisiting retinal disease. EyeNet Magazine. 2010 September.
4. Arevelo J, Garcia R, Sanchez J. The AMD/Cataract Surgery Connection. Review of Ophthalmology. 2005 December.
5. Shuttleworth GN, Luhishi EA, Harrad RA. Do patients with age related maculopathy and cataract benefit from cataract surgery? Br J Ophthalmology. 1998;82:611-6.
6. Armbrecht A, Findlay C, Aspinall P, et al. Cataract surgery in patients with age-related macular degeneration: one-year outcomes. J Cataract Refractive Surgery. 2003;29:686-93.
7. Wang J, Klein R, Smith W, et al. Cataract surgery and the 5-year incidence of late stage age-related maculopathy: pooled findings from the Beaver Dam and Blue Mountains eye studies. Ophthalmology. 2003;110:1960-7.
8. Smith B, Belani S, Ho A. Light energy, cataract surgery, and progression of age-related macular degeneration. Curr Opin Ophthalmol. 2005;16:166-9.
9. Pollack A, Marcovich A, Bukelman A, Oliver M. Age-related macular degeneration after extracapsular cataract extraction with intraocular lens implantation. Ophthalmology. 1996;103:1546-54.
10. Freeman EE, Munoz B, West SK, et al. Is there an association between cataract surgery and age-related macular degeneration? Data from three population-based studies. Am J Ophthalmol. 2003;135:849-56.
11. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch Ophthalmology. 2001;119(10);1417-36.
12. Klein B, Klein R, Moss S. Incidence of cataract surgery in the Wisconsin Epidemiolgic Study of Diabetic Retinopathy. Am J Ophthalmol. 1995;119(3):295-300.
13. Klein B, Klein R, Moss S. Prevelence of cataracts in a population-based study of patients with diabetes mellitus. Ophalmology. 1985;92(9):1191-6.
14. Baker C, Almukhtar T, Bressler N, et al. Macular edema after cataract surgery in eyes without pre-operative central-involved diabetic macular edema. JAMA Ophthalmology. 2013 July;131(7):870-9.
15. Foss R, Simons K. Vitreous in lattice degeneration of retina. Ophthalmology. 1984;91:452-7.
16. Wilkes S, Beard C, Kurland L, et al. The incidence of retinal detachment in Rochester, Minnesota, 1970-1978. Am J Ophthalmology. 1982;94:670-3.
17. Byer NE. Long-term natural history of lattice degeneration of the retina. Ophthalmology. 1989;96:1396-401.
18. Byer N. Cystic retinal tufts and their relationship to retinal detachment. Arch Ophthalmology. 1981;99:1788-90.
19. Linder B. Acute posterior vitreous detachment and its retinal complications. Acta Ophthalmology. 1966;87:1-108.
20. Benson WE. Retinal detachment: Diagnosis and Management, 2nd ed. Philadelphia: Harper & Row Publishers Inc, 1988: 4.
21. Davis MD. Natural history of retinla breaks without detachment. Arch Ophthalmology. 1974;92:183-94.
22. Colyear BH Jr, Pischel DK. Clinical tears in the retinal without detachment. Am J Ophthalmology. 1956;41:773-92.

