
History
A 51-year-old man, an old friend and neighbor, called my house at 7:30 a.m. complaining of complete loss of vision in his left eye. He said his vision was fine when he went to the bathroom at 4:00 a.m., but when he woke up at 6:00 a.m., he could not see out of his left eye despite blinking, wiping and flushing it with water.
This prompted him to call his own eye doctor. When there was no return call and no improvement in his vision, he decided to call me, hoping that I could help.
Phone triage clarified the description of his visual loss to peripheral hand motion O.S. without any pain. There was no significant ocular or systemic history. The patient denied using medications. I told him to come over to my house, where I have an office.
Diagnostic Data
Best-uncorrected visual acuity measured 20/20 O.D. and hand motion O.S. There was a grade IV afferent pupillary defect O.S. The confrontational visual field uncovered no light perception in the left eye except for a crescent-shaped area of the temporal periphery. The right eye was normal.
Color testing and brightness testing were not done. Extraocular muscles were normal. Refraction uncovered negligible refractive error, with clear retinoscopic reflexes.
Biomicroscopy with gonioscopy revealed normal anterior segment structures, normal anterior chambers and normal vitreous humors, with no evidence of iris or angle neovascularization in either eye. Goldmann applanation tonometry measured 12mm Hg O.U.
The results of the dilated fundus examination are shown in the photographs.
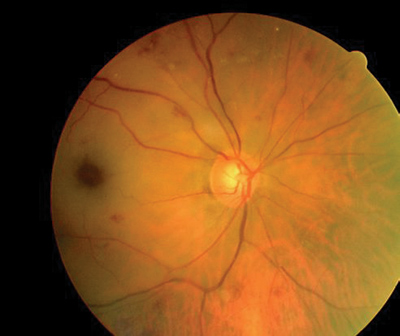 |
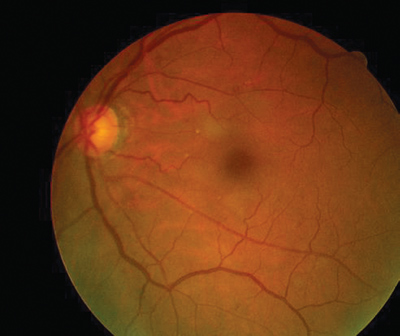 |
|
Dilated fundus photography demonstrated this appearance, O.D. (left) and O.S. (right). | |
Your Diagnosis
How would you approach this case? Does this patient require additional tests? What is your diagnosis? How would you manage this patient? What is the likely prognosis?
Discussion
Additional tests included blood pressure measurement, ophthalmodynamometry and carotid auscultation.
The differential diagnosis included idiopathic nonarteritic ischemic optic neuropathy, atypical arteritic ischemic optic neuropathy, central retinal artery occlusion, demyelinating optic neuropathy, compressive optic neuropathy and infectious/infiltrative optic neuropathy.
I referred the patient for immediate evaluation by a neuro-ophthalmologist and retinologist. I suggested laboratory studies to rule out coagulopathy, hyperviscosity, cardiac etiology, infection, inflammation, space-occupying lesion and demyelination. The suggested studies included complete blood count with differential and platelets, sphygmomanometry, fasting blood sugar, lipid panel, cholesterol, erythrocyte sedimentation rate, C-reactive protein, HLA -B27, FTA-ABS, RPR, HIV, electrocardiogram with 2-D echo, transesophogeal electrocardiogram, carotid Doppler and magnetic resonance imaging.
The neuro-ophthalmologist saw the patient in less than 24 hours and, by phone report, described the presentation as consistent with central retinal artery occlusion (CRAO). That doctor sent the patient immediately to the retinologist, who concurred with this diagnosis.
No retinal or medicinal treatment was ordered. The patient was educated about the event and informed that his vision loss would probably be permanent. The patient was instructed to obtain all the studies initially ordered and return to the neuro-ophthalmologist in two weeks.
At two weeks, the patients visual status was unchanged. The only positive laboratory study was elevated serum cholesterol (360g/dL). The final diagnosis was CRAO, presumably secondary to an embolus precipitated by high cholesterol.
Since the event, the patient has adjusted well. He views the episode as a warning sign and realizes that the outcome could have been much worse. He has made lifestyle changes that have resulted in a weight reduction of more than 60 pounds (before, at 6 foot height: 260 pounds, today: 195 pounds). His serum cholesterol (now without medication) currently measures 157g/dL.
The retinologist explained that despite the retinas perfused appearance at the initial time of examination, by the time the patient was seen, given the chronicity of events, the irreversible damage had been done. There was nothing anyone could have done. From here, we move on, taking the steps necessary to protect the patients systemic health and other eye.
CRAO is a rare event, with an estimated occurrence of 1 in 10,000 outpatient visits to eye-care professionals.1 Men are affected more commonly than women by a two-to-one ratio, with a mean age of 60 years at onset. Bilateral involvement occurs in 1% to 2% of cases.1
The site of obstruction is not usually visible on clinical examination. Most CRAOs are believed to be caused by thrombus formation at or just proximal to the lamina cribrosa. Atherosclerosis is implicated as the most common inciting event, although congenital anomalies of the central retinal artery, systemic coagulopathies or low flow states from more proximal arterial disease may also be present.1
Only 20% to 25% of CRAO cases demonstrate emboli visible in the central retinal artery or one of its branches. This suggests that embolic etiology is not frequent. Further indirect evidence opposes emboli as a frequent cause: There is less than 40% probability of finding a definitive embolic source on systemic evaluation.1
Inflammation secondary to varicella infection, optic neuritis or even orbital disease, such as mucormycosis, may cause CRAO.1 Local trauma that damages the optic nerve or blood vessels may lead to CRAO, arterial spasm or dissection, and systemic coagulopathies have been associated with both central and branch retinal arterial occlusions.1 Other rare causes include exposure to radiation, optic disc drusen and prepapillary arterial loops.1
Abrupt, painless loss of vision is the hallmark symptom of acute CRAO. Pain is unusual and suggests associated ocular ischemic syndrome. Amaurosis fugax precedes visual loss in about 10% of patients. Rarely, in cases associated with arterial spasm, a relapsing and remitting course of visual loss precedes CRAO.1,4
Examination typically reveals visual acuity of 20/800 or worse. Hand motion or light perception vision can occur but no light perception is uncommon, except in cases of an ophthalmic arterial obstruction or temporal arteritis. If a patent cilioretinal artery is present and perfuses the fovea, normal central acuity may be present. An afferent pupillary defect on the affected side is the rule.1
In the acute stage of CRAO, the anterior segment examination will appear normal, except when there is concurrent ocular ischemic syndrome with neovascularization of the iris.
In the first hours post-obstruction, the fundus may appear relatively normal (as in this case). Eventually, however, the decreased blood flow results in ischemic whitening of the retina in the territory of the obstructed artery. The macula typically manifests a cherry-red spot; this is caused by a thin nerve fiber layer, which allows visibility of the underlying choriocapillaris in contrast to the surrounding white, necrotic retina.
Splinter retinal hemorrhages on the disc are common, but more extensive retinal hemorrhages suggest an alternative diagnosis. If pallid swelling is present, temporal arteritis must be suspected. A patent cilioretinal artery results in a small area of retina that appears normal.1
After four to six weeks, the retinal whitening resolves, the optic disc develops pallor, and arterial collaterals may form on the optic disc. No foveolar light reflex is apparent, and fine changes in the retinal pigment epithelium may develop.1
Secondary ocular neovascularization is not uncommon after a CRAO. Iris neovascularization occurs in 18% of patients, with many of these eyes developing neovascular glaucoma. Panretinal photocoagulation after the vessels are detected appears to reduce the risk of neovascular glaucoma moderately.1,2
Diagnosis of CRAO is straightforward when diffuse ischemic retinal whitening is present in the setting of abrupt, painless visual loss. Fluorescein angiography may help if the diagnosis is in doubt. A delayed arm-to-retina time with a leading edge of dye visible in the retinal arteries is typical.1-4
Electroretinography characteristically reveals a decreased or absent b-wave with an intact a-wave. Visual fields show a remaining temporal island of peripheral vision. If a patent cilioretinal artery is present, a small intact central island is found as well.1
Color Doppler imaging (ultrasonography) can help determine the blood flow characteristics of retrobulbar circulation, detect calcific emboli at the lamina cribrosa and monitor blood flow changes induced by therapy. Carotid artery studies may be carried out concurrently.1-3
While systemic diseases are commonly found in patients who suffer from retinal artery obstruction, the true cause may not be clear (as in this case). About 60% of patients have concurrent systemic arterial hypertension, while diabetes is present in 25%. Systemic evaluation reveals no definite cause for the obstruction in more than 50% of affected patients. Potential embolic sources are found in fewer than 40% of cases.1-3
The most common pathogenetic association uncovered is hemodynamically significant ipsilateral carotid artery disease, which is present in about one-third of affected patients. An embolic source from the heart is present in less than 10% of patients who have CRAO; however, echocardiography and Holter monitoring should be performed, especially in younger patients. In some cases, transesophageal echocardiography is necessary to reveal embolic sources.1-3
Even though temporal arteritis is present in fewer than 5% of cases, it must be ruled out in all patients past age 50. Other rare associated systemic diseases include blood clotting abnormalities, such as antiphospholipid antibodies, protein S deficiency, protein C deficiency and antithrombin III deficiency.
No proven treatment exists for central retinal artery obstruction, but treatment strategies center on the following goals:1-4
Increase retinal oxygenation.
Increase retinal arterial blood flow.
Reverse arterial obstruction.
Prevent hypoxic retinal damage.
Theoretically, retinal oxygenation can be increased by breathing carbogen (95% oxygen, 5% carbon dioxide), although no clinical study indicates efficacy for carbogen therapy. Another method of increasing retinal arterial blood flow is by lowering intraocular pressure. This is accomplished via ocular massage, paracentesis, and the administration of ocular antihypertensive medications. Sublingual nitroglycerin, pentoxifylline (oxpentifylline), calcium channel blockers, and beta-blockers all have been used with no proof of efficacy.1
The reversal of arterial obstruction via the use of anticoagulants and fibrinolytic mediations has been reported. The utility of these mediations has not been proven by controlled clinical trials; however, anecdotal reports of success using intravenous heparin, tissue plasminogen activator, streptokinase and urokinase exist. In addition, an intra-arterial injection of tissue plasminogen activator, streptokinase or urokinase during selective catheterization of the ophthalmic artery has been attempted, with reported success in some select cases.1
Cases of CRAO associated with temporal arteritis are treated emergently with high-dose corticosteroids. Without therapy, the risk to the second eye is great. Although the first-affected eye rarely recovers, instances exist in which high-dose intravenous methylprednisolone induced visual recovery from CRAO associated with temporal arteritis.1,4
Most central retinal artery obstructions result in severe, permanent loss of vision. About one-third of patients experience some improvement in final vision in terms of presentation acuity, either with or without conventional treatment. Experimentally, if an obstruction exists in the primate retina for more than 100 minutes, complete irreversible death of the inner retina occurs. In practice, a rare patient has experienced total spontaneous recovery, even after several days of documented visual loss. Spontaneous recovery may be more common in young children.1
1. Duker JS. Retinal Artery Obstruction. In: Yanoff M, Duker JS. Ophthalmology. Philadelphia: Mosby 1999;8(17):17.1-17.17.8.
2. Ko MK, Kim DS. Posterior segment neovascularization associated with acute ophthalmic artery obstruction. Retina 2000;20(4):384-8.
3. van Norel J, van den Biesen PR, Groen GJ, van Norren D. Hold up of dye in the arm during fluorescein angiography: a quantitative demonstration. Am J Ophthalmol 2000 Apr;129(4):551-2.
4. Chan CC, Paine M, ODay J. Steroid management in giant cell arteritis. Br J Ophthalmol 2001 Sep;85(9):1061-4.

