A 53-year-old Haitian female presented with acute vision loss in her left eye that had persisted for the previous two days. She denied any pain or discomfort. There were no visual changes in the right eye.

The patient said that she underwent cataract extraction and posterior chamber IOL insertion in her left eye in 2006, and reported good vision after surgery. The patient also admitted to having a “significant health condition,” which had hospitalized her eight months earlier.
Uncorrected visual acuity measured 20/200 O.D. and 1/200 “E” O.S. With pinhole, visual acuity improved to 20/50 O.D. (no improvement O.S.). Pupils were slightly asymmetric, with a 2+ afferent pupillary defect O.S. Ocular motility was full O.U. Confrontation visual fields were normal in the right eye. Peripheral vision was intact in the left eye, but central vision was poor. The anterior segment examination was unremarkable O.U. Intraocular pressure measured 12mm Hg O.D. and 10mm Hg O.S. Additionally, we detected an early nuclear sclerotic cataract in the right eye.
The right eye was unremarkable on dilated funduscopy; however, the left eye showed significant fibrovascular membranes over the nerve and posterior pole that were most dense at the superior arcade just superior to the macula (Figure 1). We also performed optical coherence tomography (Figure 2).
Take the Retina Quiz
1. Which of the following best describes the reasoning for the patient’s poor vision?
a. Pseudoholes.
b. Macular scar.
c. Amblyopia.
d. Tractional retinal detachment.
2. Approximately how long would you suspect that the retinal lesion has been present?
a. Since birth.
b. Several hours.
c. Several days.
d. Several months.
3. What medical condition is most likely contributory to this clinical presentation?
a. Hypertension.
b. Diabetes.
c. Sickle cell anemia.
d. Age-related macular degeneration.
4. How do you explain the retinal findings?
a. Fibrous scaring secondary to an old vascular occlusion.
b. Regressed diabetic retinopathy.
c. Post traumatic.
d. Old retinal detachment.
For answers, see below.
Discussion
After examination, we further questioned the patient’s systemic health and the changes in her visual acuity. She revealed that she had been diagnosed with sickle cell disease several years ago and, in fact, had been hospitalized eight months earlier for associated complications.
At the time of her exam, her blood pressure measured 108/67mm Hg, and she denied having diabetes or any previous ocular trauma. She also admitted that her poor vision in the left eye had been bad for a few years, but it had slightly worsened recently.
Upon additional questioning, we also learned that she had seen a retinal specialist three years earlier who discovered that she had developed a peripheral vitreous hemorrhage in her left eye (likely from a neovascular sea fan). The retinal specialist documented small vascular changes superior to her left macula and noted a visual acuity of 20/40. She returned to the specialist 1.5 years later with a visual acuity of 6/200 “E” O.S., and significant posterior pole changes––including a tractional retinal detachment and fluid in the macula. This had not changed until about two days ago, when her vision decreased to 1/200.
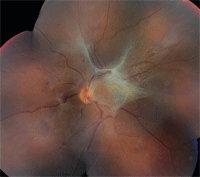
1. Fundus photo shows a fibrovascular membrane that involves the posterior pole of the left eye.
So, what caused the retinal changes in our patient? She likely had a vascular occlusion secondary to the unhealthy rigid erythrocytes associated with sickle cell disease. The retinal tissue surrounding the lesion became ischemic and vascular growth factors promoted neovascularization. These new vessels were fragile and may have even become adherent to the vitreous gel—resulting in bleeding and vitreous hemorrhage from the traction. As the hemorrhage resolved, fibrovascular membranes formed and contracted, causing a tractional retinal detachment and disruption of the integrity of the macula.
Sickle cell disease describes the inheritance of two abnormal hemogloblin genes; at least one of which is sickle hemoglobin.1 When mutant hemoglobins S or C replace one or both of the normal beta-hemoglobins, a wide spectrum of ocular manifestations may occur.2,3 Those with sickle-cell S (SS) inheritance experience severe systemic manifestations and usually minimal or mild ocular complications.2,3 Individuals with sickle-cell C (SC) disease have similar, less severe systemic complications, but may have severe retinopathy. Those with sickle-cell thalassaemia (SThal) may also experience mild anemia and severe retinopathy.3
Sickling hemoglobinopathies caused by one or a combination of abnormal hemoglobins cause the red blood cells to change in shape.3 Hypoxia and acidosis contribute to cell rigidity leading to obstruction of blood vessels.1
Posterior segment manifestations may be non-proliferative or proliferative and patients may or may not be symptomatic. Asymptomatic, non-proliferative retinal lesions include: venous tortuosity; silver-wiring of arterioles; retinal salmon patch hemorrhages; retinal iridescent spots; retinal black sunbursts; and various other vascular abnormalities affecting the choroid, macula, optic nerve and vitreoretinal interface.1-3
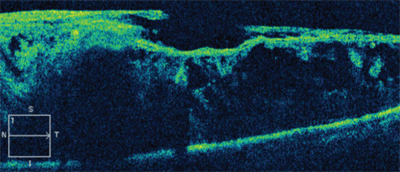
2. Optical coherence tomography scan through the patient’s left macula. What do you notice?
Other non-proliferative signs may affect vision. For example, acute central retinal artery occlusions and retinal vein occlusions are very uncommon, but macular arteriolar occlusions occur more commonly and can impact vision significantly.3 Angoid streaks also are present in 1% to 2% of patients with sickle hemoglobinopathy and may be symptomatic if changes occur in the macula.1,2
Treatment varies among patients with proliferative disease. Therapeutic or surgical intervention may be indicated in patients with bilateral disease; vitreous hemorrhage; large, elevated sea fans; rapid growth of neovascularization; or in individuals who had poor visual outcomes secondary to proliferative disease in the fellow eye.1,2
The goal of treating stage III lesions is to prevent membrane formation and progression to retinal detachment. In previously treated cases, feeder vessel photocoagulation and scatter photocoagulation have been shown to reduce the incidence of vitreous hemorrhage.2 Additionally, cryotherapy and pars plana vitrectomy may be used in advanced proliferative disease.2
We referred our patient to a vitreoretinal specialist for evaluation one week later. The specialist elected for observation. He believed that the visual potential after surgery was low, and that the tractional retinal detachment had not changed significantly over two years. He then advised the patient to return for follow-up in six months, or sooner if visual changes occur.
Thanks to Natalie A. Lambert, O.D., staff optometrist at the Bascom Palmer Eye Institute in Miami, for contributing this case.
1. Elagouz M, Jyothi S, Gupta B, Sivaprasad S. Sickle cell disease and the eye: old and new concepts. Surv Ophthalmol. 2010 Jul 8;55(4):359-77.
2. Emerson GG, Harlan JB, Fekrat S, et al. Hemoglobinopathies. In: Ryan S, Schachat P, eds. Retina. Vol. 2. Philadelphia: Elsevier-Mosby, 2006; 1429-45.
3. Kanski J, Milewski S, Damato B, Tanner V. Sickle-Cell Retinopathy. In: Diseases of the Ocular Fundus. Vol. 1. Philadelphia: Elsevier-Mosby, 2005; 63-6.
4. Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. 2009 Jun;122(6):507-12.
Answers to the Retina Quiz
1. d
2. d
3. c
4. a

