 A 56-year-old woman with a long history of primary open-angle glaucoma recently developed problems with intraocular pressure control. She was diagnosed 16 years ago and has been medicated ever since. For quite some time, her IOP was well controlled at 12mm Hg O.D. and 14mm Hg O.S. with a prostaglandin and an alpha-2 agonist.
A 56-year-old woman with a long history of primary open-angle glaucoma recently developed problems with intraocular pressure control. She was diagnosed 16 years ago and has been medicated ever since. For quite some time, her IOP was well controlled at 12mm Hg O.D. and 14mm Hg O.S. with a prostaglandin and an alpha-2 agonist.
The patient was using a number of medications for a host of other ailments, including neuropathy, osteoarthritis, hypertension, hypercholesterolemia, fibromyalgia, asthma, overactive bladder and leukemia. She was doing well with her numerous conditions, including her glaucoma, until August 2009. At that time, her IOP had risen to 22mm Hg O.D. and 30mm Hg O.S. On gonioscopy, her anterior chamber angles were open without abnormalities. Though the patient reported good compliance with her IOP-lowering medications, it was assumed that perhaps she had forgotten her drops. Therapy was unchanged, and she was scheduled to return in three weeks for an IOP check due to poor control.
Upon returning, she reported no new complaints and claimed compliance with all medications. At this visit, her IOP measured 17mm Hg O.D. and 30mm Hg O.S. She reported using the prostaglandin medication the previous evening and the alpha-2 adrenergic agonist several hours before her appointment. She reiterated that due to her numerous health problems, she was fastidiously compliant with all prescribed therapies. She was continued on all topical medications with the addition of a topical carbonic anhydrase inhibitor (CAI) to be used left eye. Due to her history of asthma, beta blockers were not considered.
She returned three weeks later for assessment of her new therapeutic regimen. She reported compliance with her therapy and no problems with the new medication. Despite the addition of the CAI, her IOP now measured 27mm Hg O.D. and 31mm Hg O.S. Her sudden failure with topical therapy was both confusing and concerning. Despite the patient’s reportedly fastidious medication use, non-compliance was nevertheless suspected.
Because maximum tolerable medical therapy seemed suddenly ineffective, she was told to discontinue all glaucoma medications temporarily to re-establish baseline values. If she stopped all medications and her IOP did not further elevate, then compliance was the issue. She was scheduled for a one-week follow-up and told to call immediately should anything change.
She presented emergently three days later complaining of dilated pupils and blurry vision at near. While her visual acuity was unchanged, her pupils were both dilated and sluggishly reactive without afferent defect. Her untreated IOP was now 34mm Hg O.D. and 43mm Hg O.S. Again, gonioscopy revealed normal open anterior chamber angles.
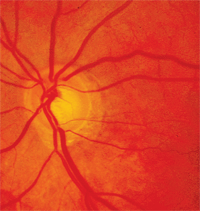
Glaucomatous optic disc damage can occur from intraocular pressure elevation associated with systemic medication use.
The patient’s new complaints clarified the situation. The blurred vision and mydriasis seemed to point towards medication overdosage and toxicity. Her primary care physician had increased the dosage of two medications, Vesicare (solifenacin succinate, Astellas Pharmaceuticals) and Neurontin (gabapentin, Pfizer), approximately one month before the problems with IOP control began. She reported that she previously had been using 600mg of Neurontin and 5mg of Vesicare. Her physician had increased the Neurontin to 900mg and the Vesicare to 10mg.
Inspection of medications identified the error. When her physician increased her Vesicare dosage to 10mg, he prescribed it in 10mg tablet form—which looked identical to the 5mg tablets that she had been using. She thought that the new prescription was the same 5mg concentration and that she should take two tablets for a total of 10mg. Inadvertently, she had been taking two tablets of what she thought was 5mg when, in reality, she was taking two 10mg tablets. Her dosage of Vesicare had accidentally been quadrupled overnight, and this is likely what led to her problems. This case leads us to this month’s topic: systemic medications that can increase intraocular pressure.
What is Vesicare?
Vesicare is used to treat overactive bladder and its symptoms, including urinary incontinence, urgency, and urinary frequency by reducing muscle spasms of the bladder and urinary tract. It is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergically mediated functions, including contractions of urinary bladder smooth muscle and stimulation of salivary secretion, as well as accommodative and pupillary functions.
The most common adverse events reported in patients treated with Vesicare are dry mouth and constipation. However, overdosage with Vesicare can potentially result in severe anticholinergic effects, such as fixed, dilated pupils and accommodative dysfunction. Increased intraocular pressure has also been noted.1,2
What is Neurontin?
Neurontin is specifically indicated for the management of post-herpetic neuralgia in adults. This gamma-aminobutyric acid (GABA) analogue was originally developed for the treatment of epilepsy. Neurontin is widely used to relieve pain—especially neuropathic pain.3
Additionally, Neurontin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in patients over 12 years of age who have epilepsy. Neurontin is also commonly used to treat neuralgic pain from other causes. Further, it has been found to be effective in prevention of frequent migraine headaches and nystagmus.4,5 Blurred vision and glaucoma have been reported rarely with Neurontin use.6
Other Possible Associations
Spiriva (tiotropium bromide inhalation powder, Pfizer/Boehringer Ingelheim) is an anticholinergic medication indicated for the long-term, once-daily maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. As an anticholinergic drug, Spiriva may lead to mydriasis and initiate an angle-closure glaucoma attack in patients with potentially occludable angles. Additionally, it may worsen symptoms and signs associated with prostatic hyperplasia or bladder-neck obstruction and should be used with caution in patients with any of these conditions.7
Cymbalta (duloxetine, Eli Lilly) is a serotonin-norepinephrine reuptake inhibitor indicated for the treatment of major depressive disorder, generalized anxiety disorder, neuropathic pain associated with diabetic peripheral neuropathy, and fibromyalgia. In clinical trials, Cymbalta was associated with an increased risk of mydriasis; it should be used cautiously in patients with potentially occludable anterior chamber angles.
After identifying the potential overdose, the patient contacted her primary care physician, who immediately adjusted her dosages. For her elevated IOP, a different prostaglandin as a sole agent was prescribed.
At a progress evaluation one week later, her IOP was much improved: 16mm Hg O.D. and 24mm Hg O.S. She reported that her near blur and dilated pupils were improved, but not completely resolved. Over several weeks, however, these symptoms disappeared. The alpha-2 agonist was added again to the regimen, and the patient continues to do well.
This case was notable in that a patient being treated for glaucoma had a sudden loss of IOP control. In most cases, noncompliance is the probable cause; however, this patient maintained that she adhered to all medication schedules. Fortunately, other clinical signs and symptoms appeared that indicated drug reactions.
In cases of unexpected IOP rise, carefully examine your patients’ systemic medications as possible causes.
1. Wagg A, Wyndaele JJ, Sieber P. Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis. Am J Geriatr Pharmacother. 2006 Mar;4(1):14-24.
2. Cardozo L, Lisec M, Millard R, et al. Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004 Nov;172(5 Pt 1):1919-24.
3. Baillie JK, Power I. The mechanism of action of gabapentin in neuropathic pain. Curr Opin Investig Drugs. 2006 Jan;7(1):33-9.
4. Mathew NT, Rapoport A, Saper J, et al. Efficacy of gabapentin in migraine prophylaxis. Headache. 2001 Feb;41(2):119-28.
5. Choudhuri I, Sarvananthan N, Gottlob I. Survey of management of acquired nystagmus in the United Kingdom. Eye (Lond). 2007 Sep;21(9):1194-7.
6. Tsiropoulos I, Andersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepidemiol Drug Saf. 2009 Jun;18(6):483-91.
7. Oksuz H, Tamer C, Akoglu S, et al. Acute angle-closure glaucoma precipitated by local tiotropium absorption. Pulm Pharmacol Ther. 2007;20(6):627-8.

