In
A wide variety of clinical conditions make up what we call secondary glaucomas. One thing that they often have in common is a blockage of the trabecular meshwork that eventually leads to an increase in intraocular pressure (IOP).
But, increased venous pressure leads to a secondary glaucoma that is NOT an obstruction of the trabecular meshwork. Other causes are trabeculitis and steroid-induced glaucoma.
Unfortunately, because a spike in IOP is often the first symptom detected, secondary glaucomas may be misdiagnosed as POAG. An accurate differential diagnosis requires a comprehensive examination of the anterior and posterior segments. Such an exam must include gonioscopy. We cannot skip this crucial step on any glaucoma suspect, nor can we rely solely on a slit lamp examination to detect a narrow angle, ocular inflammation, new vessel formation or sign of trauma.
Here, we will discuss the most commonly encountered secondary glaucomas: pigmentary glaucoma (PG), exfoliative glaucoma (XFG), uveitic glaucoma (UG), and steroid-induced glaucoma. We will also review a serious secondary angle-closure glaucoma: neovascular glaucoma (NVG).
Pigmentary Glaucoma
Pigmentary glaucoma (PG) was first reported in 1949 as a constellation of findings, such as pigment deposition on the corneal endothelium, lens and trabecular meshwork.2 Researchers believed that PG was caused when pigmentary granules from the iris pigment epithelium came loose and obstructed the trabecular meshwork.
By 1979, researchers believed that the disease was caused by packets of lens zonules rubbing against the peripheral iris, thus freeing pigment. This was evidenced by characteristic transillumination defects in the iris.3 In 1992, researchers suggested that posterior bowing of the peripheral iris was caused by reverse pupillary block.4 This, in turn, led to the idea that a laser peripheral iridotomy (LPI) could remove the mechanical component of the disease by relieving the concavity.
Not everyone who has deposits of pigmentary granules, or pigmentary dispersion syndrome (PDS), develops PG. About 2.5% of the population has PDS, and some estimate that 50% of PDS patients will convert to PG at some point in their disease.5,6 A recent population study estimated that 10% of those develop PG within five years of diagnosis and 15% within 15 years.7 White males under the age of 45 who have significant myopia are especially at risk for converting from PDS to PG.6
Researchers doubt that pigmentary obstruction is the key factor in development of increased IOP. An acute release of pigment can cause a temporary rise in IOP, but the trabecular cells have a high capacity for processing and eliminating these pigmentary granules.8,9
Histopathological studies point to several other processes that might cause the disease. For example, in patients who have PG, pigment-containing cells die in the trabeculum, and fusion of the lamellae occurs. This eventually leads to loss of intertrabecular spaces and decreased outflow.10
An early indicator of pigment dispersion syndrome (PDS) is a spindle-shaped, vertical deposit of pigment in the cornea known as Krukenberg"s Spindle.
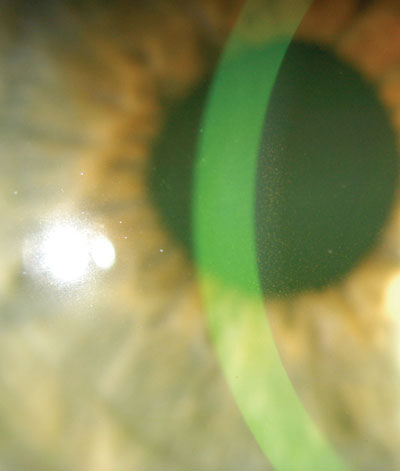
Patients who have PG or PDS and ocular hypertension should be managed with medication, laser treatment or surgery, depending on the IOP and disease severity. Drug therapies include prostaglandin analogues (PGA), alpha-agonists and beta-blockers.
When drugs fail, laser trabeculoplasty becomes an option. Because it requires pigment in the angle to have its thermal effect, argon laser trabeculoplasty (ALT) has long been considered the primary surgical treatment for PG. Another option, selective laser trabeculoplasty (SLT), applies less energy to the surgical site and induces less collateral damage.11 The diminished energy levels support the theoretical advantage of repeatable surgeries. Patients might undergo scheduled cleanings with SLT.
There has been much debate regarding the efficacy of LPI for treating PDS and PG. The jury is still out on this question, but in my opinion, LPI should be considered for younger patients, which is the group that experiences the most active pigment liberation. If we had a method to predict which patients would progress from PDS to PG, then I believe prophylactic LPI for such patients would be reasonable as well.
Exfoliation Glaucoma
This secondary glaucoma is associated with a systemic condition called exfoliation syndrome (XFS). This syndrome is characterized by dysfunctional basement membranes in multiple cell types, including those in the trabecular meshwork.
More recently, XFS has been linked to an abnormal protein synthesis that creates a trabecular meshwork-blocking extracellular matrix synthesis.12 This obstruction can result in elevated IOP and conversion to exfoliation glaucoma (XFG). Because exfoliation syndrome can cause iridolenticular contact, liberated pigment may be deposited into the trabecular meshwork as well.

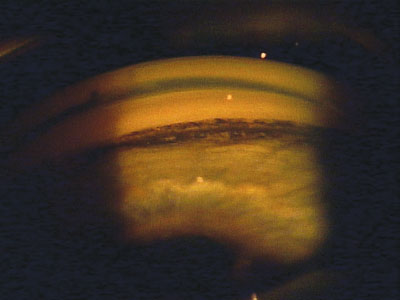
Pigment deposits in the trabecular meshwork and angle as seen by gonioscopy.
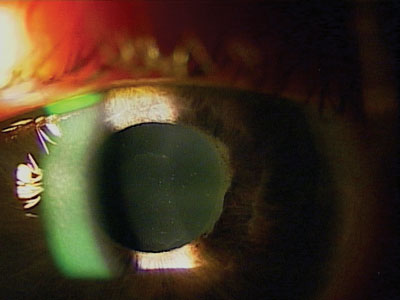
The classic signs of XFS include a sugar-like frosting on the anterior lens capsule and white flaky material on the pupillary margin.13 Occasionally, the exfoliation can be seen with gonioscopy as well.
About 25% of patients who have XFS exhibit ocular hypertension. Of these, 30% to 40% go on to develop XFG.14-16 Patients who have established XFG tend to exhibit higher baseline IOP and more severe disc and visual field damage at diagnosis than those who have POAG. This is probably due to the higher diurnal fluctuations seen in XFG.17
Additionally, XFS and the frictional wear against the iris make for weakened lens zonules, which may be a factor in patients being considered for cataract surgery.
Medical management of XFG is similar to that of POAG. But, XFG is particularly resistant to drug therapy and difficult to control. Prostaglandins should still be your first-line therapy, but adjunctive use of miotics might be necessary. When tolerated, miotics have some advantages. For example, pilocarpine increases trabecular outflow and minimizes iridolenticular contact, thus reducing migration of pigment and exfoliative material to the trabecular meshwork. Of course, potential side effects limit the feasibility of miotics for many patients. Side effects include headache and brow ache, to name a few.
Indications for laser intervention are similar to PG; since the outflow obstruction of XFG is chronic, a potentially repeatable procedure such as SLT would be beneficial.
A sugar-like frosting on the anterior lens capsule and white flaky material on the pupillary margin may indicate exfoliation syndrome (XFS).

Uveitic Glaucoma
Uveitic glaucoma (UG) presents a two-pronged and very serious problem: inflammatory damage to the trabecular meshwork and uvea coupled with steroid-induced ocular hypertension. It is perhaps the most difficult glaucoma to manage because we must simultaneously treat chronic inflammation and elevated IOP.
The pathophysiology of UG remains unclear. Inflammation causes synechial damage in the angle, most often in the form of peripheral anterior synechiae (PAS). Although PAS is not thought of as the primary cause of high IOP, increased PAS means a higher likelihood that IOP has been impacted. Inflammation causes changes in aqueous composition, production, and outflow, but researchers believe that most of IOP response can be attributed to the corticosteroids used to treat the underlying uveitis.17,18 Corticosteroids raise IOP by causing a decrease in aqueous outflow and an increase in aqueous production.19
According to studies, the incidence of secondary glaucoma in uveitis is between 10% and 20%.20,21 But, some of this data is based on diagnosing glaucoma solely as elevated IOP, not as visual field loss or optic nerve damage.
One of the most common causes of secondary glaucoma in uveitis is idiopathic anterior uveitis. Several subtypes of uveitis yield high rates of secondary glaucoma. For example, herpes simplex keratouveitis progresses to glaucoma in 54% of cases, and herpes zoster uveitis progresses in 38%.22 Fuchs heterochromic cyclitis and sarcoid- related uveitis also yield high rates of glaucoma progression.
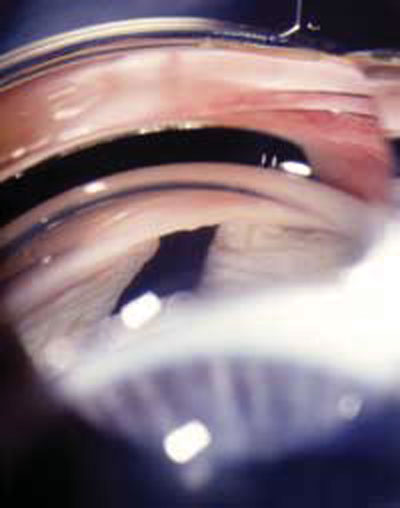
Currently, patients who have UG are responding to topical glaucoma medications, but success rates for any one drug are highly variable and unpredictable. Use caution when prescribing prostaglandins because a small percentage of uveitis patients will experience increased inflammation. Cystoid macular edema (CME) is a common complication of chronic pan-uveitis, and there have been anecdotal (but reliable) reports of CME developing in uveitis patients taking prostaglandins, especially in the presence of pseudophakia.23-25
Interactions with topical and oral nonsteroidal anti-inflammatory drugs (NSAIDs) may help explain unpredictable responses to glaucoma medications. Researchers speculate that topical NSAIDs block part of the feedback loop that regulates prostaglandin release. A study investigating the effect of brimonidine and latanoprost with concurrent oral indomethacin found a partial reduction in the efficacy of brimonidine, but no effect on that of latanoprost.26 When used concurrently with topical NSAIDs, however, latanoprost demonstrated a partial reduction in efficacy.27 This is a concern in uveitis patients who are being treated by a rheumatologist.
In the acute stages of UG, give preference to agents that have a short onset of action, such as topical beta-blockers and alpha-agonists. Again, PGAs are not contraindicated, but they usually take two to three weeks to be effective and may exacerbate inflammation. Surgical management generally yields less than 50% success at five years, although an anti-metabolite used adjunctively with trabeculectomy will improve success modestly.28
Controlling inflammation is crucial. Full-strength steroids are preferred for the initial management of uveitis. Once the inflammation is under control, a slow taper and steroid replacement should then follow.
Selective Laser Trabeculoplsaty (SLT) is an effective treatment for both pigmentary glaucoma and exfoliative glaucoma because it is a potentially repeatable procedure.
After uveitis is controlled with high-dose topical steroids, consider switching to a non-ketone steroid, such as loteprednol. This ester-based agent has no propensity to raise IOP and is well suited for protracted tapering dosage.
Neovascular Glaucoma
Neovascular glaucoma (NVG) is another glaucoma subtype that is very difficult to treat. It develops secondary to ischemic processes in the retina and anterior segment. Early recognition of these processes increases the chance of preventing vision loss.
Causes of NVG include retinal vein occlusions, proliferative diabetic retinopathy, retinal arterial occlusions, and carotid insufficiency. The end result is the development of a fibrovascular membrane that obstructs the trabecular meshwork.
The hallmark sign of full-blown NVG is rubeosis iridis, or iris neovascularization. The iris is the most anterior tissue to be affected by neovascularization, but earlier detection is possible by examining the retina, the optic nerve or the angle. To intervene in a timely manner, perform funduscopy and gonioscopy on patients who have a history of vascular occlusive disease or proliferative diabetic retinopathy.
The goals of treatment are to reduce IOP and eradicate the cause of neovascularization. Since most neovascularization originates in the retina, panretinal photocoagulation (PRP) is often used to decrease the hypoxic stimulus and induce regression of neovascularization. But, once the disease has transversed and reached the angle, the fibrovascular membrane obstruction in the meshwork will persist.
Exfoliation seen in the angle via gonioscopy.
Tube-shunt procedures hold promise as secondary options.29 Trans-scleral cyclophotocoagulation (TCP) and endocyclophotocoagulation (ECP) are also options for advanced or refractory cases. Vascular endothelial growth factor (VEGF) likely enables the neovascularization process, and current studies are investigating the uses of anti-VEGF drugs in the management of NVG.
Having removed the neovascular stimulus, our aim of treatment turns to suppressing aqueous production. Aqueous-suppressing drugs include beta-blockers, alpha-agonists, and topical carbonic anhydrase inhibitors. Oral carbonic anhydrase inhibitors are also useful for treating NVG. PGAs should be used cautiously; there is a risk of inflammation, they do not suppress aqueous production, and they will vary in results.
The secondary glaucomas are a complex group of diseases that require careful diagnostic considerations. Skillful gonioscopy can detect these forms of glaucoma and is essential for successful diagnosis and long-term management. Recognition of the underlying causes of inflammatory and neovascular glaucoma can help in the triage efforts of multidisciplinary clinicians.
Dr. Ben Gaddie serves on the faculty at Northeastern State University College of Optometry and is also in private practice in
1. Teikari JM, ODonnell J. Epidemiologic data on adult glaucomas. Data from the Hospital Discharge Registry and the Registry of Right to Free Medication. Acta Ophthalmol (Copenh) 1989 Apr;67(2):184-91.
2. Sugar HS, Barbour FA. Pigmentary glaucoma. A rare clinical entity. Am J Ophthalmol 1949;32:90-2.
3. Scheie HG, Fleischhauer HW. Idiopathic atrophy of the epithelial layers of the iris and ciliary body; a clinical study. AMA Arch Ophthalmol 1958 Feb;59(2):216-28.
4. Karickhoff JR. Pigmentary dispersion syndrome and pigmentary glaucoma: a new mechanism concept, a new treatment, and a new technique. Ophthalmic Surg 1992 Apr;23(4):269-77.
5. Ritch R, Steinberger D, Liebmann JM. Prevalence of pigment dispersion syndrome in a population undergoing glaucoma screening. Am J Ophthalmol 1993 Jun 15;115(6):707-10.
6. Farrar SM, Shields MB, Miller KN, Stoup CM. Risk factors for the development and severity of glaucoma in the pigment dispersion syndrome. Am J Ophthalmol 1989 Sep 15;108(3):223-9.
7. Siddiqui Y,
8. Matsumoto Y, Johnson DH. Trabecular meshwork phagocytosis in glaucomatous eyes. Ophthalmologica 1997;211(3):147-52.
9. Buller C, Johnson DH, Tschumper RC. Human trabecular meshwork phagocytosis. Observations in an organ culture system. Invest Ophthalmol Vis Sci 1990 Oct;31(10):2156-63.
10. Gottanka J, Johnson DH, Grehn F, Lutjen-Drecoll E. Histologic findings in pigment dispersion syndrome and pigmentary glaucoma. J Glaucoma 2006 Apr;15(2):142-51.
11.
12. Schlotzer-Schrehardt U, Kuchle M, Naumann GO. Electron-microscopic identification of pseudoexfoliation material in extrabulbar tissue. Arch Ophthalmol 1991 Apr;109(4):565-70.
13. Roth M, Epstein DL. Exfoliation syndrome. Am J Ophthalmol 1980 Apr;89(4):477-81.
14. Kozart DM, Yanoff M. Intraocular pressure status in 100 consecutive patients with exfoliation syndrome. Ophthalmology. 1982 Mar;89(3):214-8.
15. Henry JC, Krupin T, Schmitt M, et al. Long-term follow-up of pseudoexfoliation and the development of elevated intraocular pressure. Ophthalmology 1987 May;94(5):545-52.
16. Ringvold A, Blika S, Elsas T, et al. The middle-Norway eye-screening study. II. Prevalence of simple and capsular glaucoma. Acta Ophthalmol (Copenh) 1991 Jun;69(3):273-80.
17. Epstein DL, Hashimoto JM, Grant WM. Serum obstruction of aqueous outflow in enucleated eyes. Am J Ophthalmol 1978 Jul;86(1):101-5.
18. Toris CB, Pederson JE. Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci 1987 Mar;28(3):477-81.
19. Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol 1965 Apr;4:198-205.
20. Merayo-Lloves J, Power WJ, Rodriguez A, et al. Secondary glaucoma in patients with uveitis. Ophthalmologica. 1999;213(5):300-4.
21. Takahashi T, Ohtani S, Miyata K, et al. A clinical evaluation of uveitis-associated secondary glaucoma. Jpn J Ophthalmol 2002 Sep-Oct;46(5):556-62.
22. Miserocchi E, Waheed NK, Dios E, et al. Visual outcome in herpes simplex virus and varicella zoster virus uveitis: a clinical evaluation and comparison. Ophthalmology 2002 Aug;109(8):1532-7.
23. Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patients. Ophthalmology 1998 Feb;105(2):263-8.
24. Smith SL, Pruitt CA, Sine CS, et al. Latanoprost 0.005% and anterior segment uveitis. Acta Ophthalmol Scand 1999 Dec;77(6):668-72.
25. Gaddie IB, Bennett DW. Cystoid macular edema associated with the use of latanoprost. J Am Optom Assoc 1998 Feb;69(2):122-8.
26. Sponsel WE, Paris G, Trigo Y, et al. Latanoprost and brimonidine: therapeutic and physiologic assessment before and after oral nonsteroidal anti-inflammatory therapy. Am J Ophthalmol 2002 Jan;133(1):11-8.
27. Kashiwagi K, Tsukahara S. Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost. Br J Ophthalmol 2003 Mar;87(3):297-301.
28. Stavrou P, Murray PI. Long-term follow-up of trabeculectomy without antimetabolites in patients with uveitis. Am J Ophthalmol 1999 Oct;128(4):434-9.
29. Mermoud A, Salmon JF, Alexander P, et al. Molteno tube implantation for neovascular glaucoma. Long-term results and factors influencing the outcome. Ophthalmology 1993 Jun;100(6):897-902.

