
Uveitis affects individuals of all ages, genders and races. It is recognized as an entity unto itself; however, it is not one single disease. Uveitis is actually a manifestation of diverse conditions that result in an inflammatory response within the uveal tract: the iris, ciliary body and choroid.
Acute anterior uveitis is the most common form encountered and is usually managed in the primary eye-care setting.1 But, chronic forms of uveitis associated with systemic disease often require comanagement with a specialist. Following treatment, the ocular condition generally resolves as the systemic condition is treated.
In all cases of uveitis, aggressive treatment is essential to control the inflammatory process and minimize tissue damage, scarring and potential structural alterations. Complications, such as secondary glaucoma from posterior synechiae, angle closure and cystoid macular edema, can cause permanent vision loss.
Primary-care optometrists need to understand the full range of therapeutic options in order to best comanage and effectively treat their uveitic patients.
In particular, cases of refractory uveitis have been shown to respond to new treatment modalities, including systemic immunosuppressive and immunomodulatory agents.
This paper covers noninfectious uveitis associated with systemic inflammatory or autoimmune diseases, selected immunologic mechanisms and the rationale for immunomodulatory therapy.
Classification
Knowing the type of uveitis helps us identify the cause and decide upon the appropriate treatment. So, how should we classify uveitis? The International Uveitis Study Group (IUSG) bases its widely used classification system on the anatomic location of the inflammation, regardless of cause.2
Other classification systems define uveitis according to the type of inflammation (granulomatous, non-granulomatous), laterality (unilateral, bilateral), duration of inflammation (acute, chronic) and other causative factors (infectious, autoimmune, systemic or neoplastic diseases).3
In 2005, the Standardization of Uveitis Nomenclature (SUN) Working Group was formed to review the terminology of uveitis.4 The purpose of forming a consensus regarding terminology was to promote comparative studies and data analysis from multiple study centers. The SUN Working Group endorsed the IUSG anatomic classification system and added a few clarifying points.4
Based on anatomic location of inflammation, uveitis can be categorized as:
Anterior uveitis. Inflammation of the anterior chamber includes iritis, iridocyclitis and anterior cyclitis.
Intermediate uveitis. Inflammation of the vitreous is the major site of involvement, including pars planitis, posterior cyclitis and hyalitis.
Posterior uveitis. The choroid and/or the retina (chorioretinitis) become inflamed.
Panuveitis. Inflammation is not predominant at any one site; rather, two or more are involved, such as the anterior chamber, vitreous and retina or choroid.
The terms acute and chronic have several applications referring to either the onset of uveitis, the duration of the condition or the entire course of the uveitis. According to the SUN Working Group, however, these terms should describe the clinical course of uveitis:
Acute. Such an episode is characterized by sudden onset and limited duration.
Chronic. Chronic uveitis is persistent and tends to relapse less than three months after discontinuing treatment.
Recurrent. This includes repeated episodes separated by periods of inactivity not requiring treatment longer than three months in duration.4
Pathogenesis
Uveitis is an inflammatory condition intended to protect the eye by eliminating the potentially harmful inciting agent. It can be instigated by infectious agents, trauma, or underlying systemic disease. But, the inciting cause is unknown in most cases.
Although the inflammatory response is a defense mechanism, it sometimes leads to detrimental alteration of ocular structure or function. So, under normal conditions, the eye seeks to avoid an inflammatory confrontation; instead, it maintains an immunosuppressive environment. This is known as immune privilege. The loss of immune privilege may play a central role in non-infectious or autoimmune uveitis.5-7 Research using rodent models for uveitis supports this idea.7
Immune privilege protects the eye from the consequences of an active inflammatory response. Organs with this unique ability rely on an immune system that avoids the responses that would impair their function. These organs include the brain, with its limited regenerative capacity, and the pregnant uterus.5
The factors that contribute to immune privilege in the eye include the anatomy of ocular structures and tissue-specific cell surface and secreted proteins (see Features Contributing to Immune Privilege).5,6 Together, these restrict either the delivery or recognition of an antigen both to and by the ocular structures, creating the immunosuppressive environment.5,6
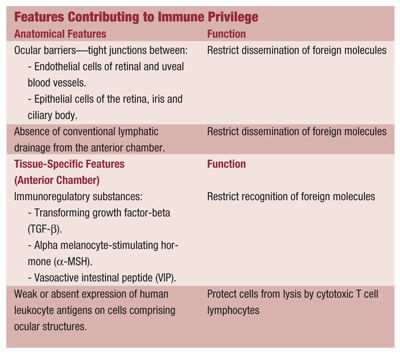
The immunologic mechanisms of uveitis associated with systemic or autoimmune diseases are multifactorial, and the exact process remains unclear. But, known factors include genetic susceptibility, previous exposure to infectious agents and loss of immune privilege.
Genetic susceptibility to immune-mediated diseases is linked to specific human leukocyte antigen (HLA) subtypes. Human leukocyte antigens are associated with immune responses and play a prominent role in organ transplant rejection, vulnerability to autoimmune diseases and susceptibility to infectious diseases.8
HLA molecules are proteins (peptides) that are present on the surfaces of many cells of the body. They represent the product of genes comprising the major histocompatibility complex (MHC) on chromosome 6.9 Human leukocyte antigens function to present antigen to lymphocytes.
Lymphocytes, in turn, recognize the HLA proteins of other cells, determining self vs. non-self, or foreign cells. So, the HLA system helps regulate the nature and strength of immune responses.9
As the HLA system is so important to immune responses, genetic susceptibility to immune-mediated diseases is linked to specific HLA subtypes. One of the best-known HLA-disease associations involves HLA-B27, which consists of 24 subtypes that vary in prevalence among different ethnic groups. Some subtypes carry a stronger risk for HLA-B27-associated disease, namely the seronegative spondyloarthropathies, such as ankylosing spondylitis.3,7,9
The uveitis associated with ankylosing spondylitis is usually a unilateral acute anterior type. Disease susceptibility in individuals positive for HLA-B27 is complex and likely involves a specific HLA-B27 subtype in combination with other genetic and environmental factors.7-9
Extensive research has led to several hypotheses that attempt to explain the mechanisms linking HLA-B27 and its associated inflammatory diseases.8,9 They include:
Molecular mimicry, the resemblance of antigenic microbial proteins to host (self) tissue. An immune response against a microbial component may cross-react with a similar host structure. A T lymphocyte-mediated response may occur against a self peptide found only in joint tissue (arthritogenic peptide) or ocular tissue (uveitogenic peptide).
Abnormal forms of HLA-B27, which may present microbial or self proteins to T cell lymphocytes, triggering an immune response.
HLA-B27 breakdown products, which may serve as autoantigens and initiate an autoimmune response.
Another HLA-disease association exists: HLA-A29 is present in more than 90% of individuals with birdshot retinochoroidopathy, a posterior uveitis characterized by multiple, hypopigmented choroidal lesions.7,10 Also, HLA-B51 has a strong link with Behets disease, a multisystem autoimmune condition characterized by recurrent uveitis, skin lesions, and oral and genital ulcers.7
Although the inciting stimulus for uveitis in systemic immune disease is poorly understood, it has been suggested that a breakdown in immune privilege allows T cell lymphocytes to react against self antigen.6 T cell lymphocytes become active and proliferate; the newly formed daughter cells differentiate into T helper (CD4+) lymphocytes.
In general, T helper cells can be characterized as either T helper cell type 1 or type 2 (Th1 or Th2), based on the cytokines they produce. Usually, the two T cell subtypes regulate each other in a balanced manner. Th2 responses are associated with antibody responses, protection from autoimmune disease and, in disease states, allergy. Th1 responses, on the other hand, account for cell-mediated inflammatory reactions, delayed-type hypersensitivity and tissue injury in autoimmune diseases.5,7,8
In determining the type of inflammatory response, the two most important factors to analyze are the inciting cause and the cytokines produced.11 For example, infectious uveitis usually shows an initial neutrophil responseand with a chronic course, lymphocytes will predominate. These cells produce cytokines within the eye that dictate the ocular response to infection. Non-infectious uveitis is
typified by Th1 cytokines produced in the eye and present in peripheral blood.12 But, similarities in the immune responses of infectious, autoimmune and idiopathic uveitides may exist.11
Animal models for anterior and posterior uveitis support the important role of Th1 lymphocytes and their proinflammatory cytokines. Elevated levels of tumor necrosis factor-alpha (TNF-a), interleukin-2 (IL-2) and interferon-gamma (IFN-g) have been detected in animal and human studies of uveitis.7,11,13,14 These cytokines regulate immune responses, cell activation and recruitment of inflammatory cells.11,14 This, in turn, causes breakdown of the blood ocular barrier, which results in vascular permeability and leakage of both protein (flare) and cells into the anterior chamber or other affected sites.
Our understanding of cytokines has led to the development of therapeutic agents designed to neutralize their action. These immunomodulatory and biologic agents were developed for treating autoimmune diseases, such as rheumatoid arthritis and inflammatory bowel syndrome, but have recently been used in the treatment of refractory cases of uveitis.
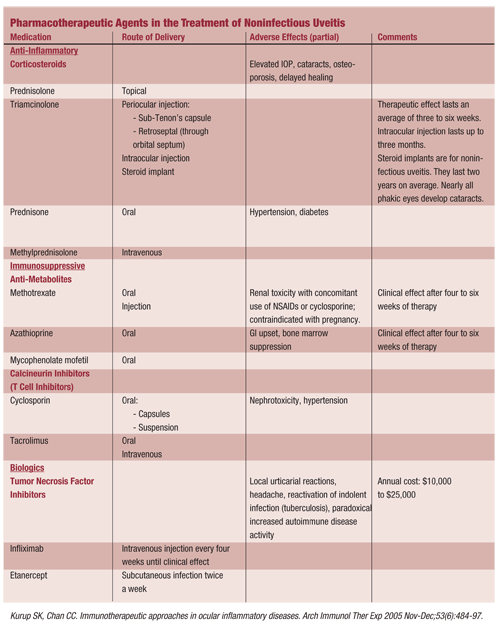
Aggressive treatment of uveitis aims to control the inflammatory response to minimize tissue damage and scarring. Cycloplegia and topical corticosteroids are the first line of treatment; the U.S. Food and Drug Administration (FDA) approved these drugs for use in treating uveitis. Corticosteroids can also be given locally as periocular or intravitreal injections or systemically (either orally or intravenously).
If systemic corticosteroids are ineffective, or if there are concerns regarding side effects, then immunosuppressive therapy may be considered by the managing systemic disease practitioner. The managing clinician may use T cell inhibitors, such as cyclosporine and tacrolimus, to inhibit the production of cytokines, or antimetabolites, such as azathioprine, methotrexate and mycophenolate mofetil.13,15,16 Cytokine inhibitors bind to cytokine receptors or ligands to block selected cytokines that are produced in the inflammatory response.
Here are the current and potential drugs to treat uveitis:
Corticosteroids. Corticosteroids are used to control the acute phase of uveitis. Corticosteroids act by binding to the glucocorticoid receptor on the cell surface, migrating to the nucleus and modulating several genes involved in inflammatory and immune responses. Steroids reduce the recruitment of monocytes, constrict blood vessels, decrease vascular permeability and down-regulate arachidonic acid metabolism.
A lack of response to topical steroids warrants comanagement with a uveitis specialist and consideration of injectable or oral steroids. If the uveitis is recurrent, chronic, or has a poor outcome with corticosteroid monotherapy, then immunosuppressive drugs may be indicated.15,16
These drugs can be used alone, in combination with another immunosuppressive agent or with a reduced corticosteroid dose. Weigh the benefits of corticosteroid therapy against the potential adverse effects (see Pharmacotherapeutic Agents in the Treatment of Noninfectious Uveitis); corticosteroids can be independent predictors of mortality in patients with systemic inflammatory disease.15,17
Cycloplegics. Cycloplegic agents, such as atropine and scopolamine, are used with topical corticosteroids as the first line of anterior uveitis treatment. They reduce anterior uveal inflammation by decreasing the vascular permeability associated with the inflammatory response, thereby reducing the leakage of cells and protein into the anterior chamber.
In addition, these drugs relax ciliary muscle spasm and decrease posterior synechiae by dilating the pupil, which decreases contact between the iris and lens.18
Antimetabolites. Antimetabolites compete with a particular metabolite required for nucleotide synthesis. Methotrexate is a folic acid analog that binds to and blocks the enzyme necessary for the conversion of dietary folic acid to the metabolically active form. Interference with cell division and inhibition of T and B cells follows.
Methotrexate is a first-line immunosuppressive drug used off-label to treat various ocular inflammatory conditions, and low-dose, long-term usage is common in the management of rheumatoid arthritis and recurrent uveitis.6,13,15,16
Methotrexate has been added to other treatment regimens, such as concomitant corticosteroid use, which allows control of inflammation with a reduction in corticosteroid dose.13
Azathioprine is a purine analog approved for use in organ transplantation and for treatment of malignancies and autoimmune disorders. It has also been used off-label to treat uveitides that have a poor response to or adverse side effects from steroid therapy.13,15 Azathioprine interferes with DNA synthesis and cell division, with greater effect on T cells than B cells.13,15
Mycophenolate mofetil (MMF) is a selective purine synthesis inhibitor, which affects a pathway preferentially used by T and B lymphocytes to inhibit their proliferation.6,15,16,19 It is a more specific inhibitor than azathioprine. In a limited study of 17 children, MMF was effective in treating chronic noninfectious uveitis, allowing oral prednisolone to be discontinued or reduced to a low maintenance dose.17,20
Calcineurin inhibitors. Cyclosporine is a natural product of fungi that functions as a calcineurin inhibitor. It interferes with the intracellular signaling pathways of
T cells and the production of cytokines. Inhibition of signaling pathways occurs after antigen recognition. Tacrolimus is also a metabolic product of fungi. It is structurally different from cyclosporine, but it has a similar mechanism of actionit disrupts T cell activation and cytokine production.6,13,15,16
Biologics. Biologic agents include monoclonal antibodies (mAb) and recombinant forms of natural inhibitory molecules. Infliximab and etanercept are inhibitors of the inflammatory cytokine, tumor necrosis factor-alpha (TNF-a). TNF-a inhibitors have also been useful in the treatment of rheumatoid arthritis, juvenile rheumatoid arthritis, ankylosing spondylitis and Crohns disease. Limited studies have shown them to be effective in the treatment of uveitis.6,13,15,16
Infliximab is a chimeric (murine-human) monoclonal antibody directed against the soluble and membrane-bound forms of TNF-a. Etanercept is a genetically engineered fusion protein that binds both TNF-a and TNF-b, preventing interaction with their natural receptors on cell surfaces.6,13,15,16 Although there are no comparative, randomized trials of the TNF-a inhibitors, limited studies indicate that antibody-based TNF-a inhibition (infliximab) may be more effective in the treatment of uveitis than the receptor-blocking agents (etanercept).13,14,21,22
In most cases, the cause of uveitis is unknown; however, there is evidence for immune-mediated mechanisms, as many patients have associated systemic disease. With subsequent research and better understanding of immune cells and cytokines, new treatment modalities are being developed. Already, immunosuppressive drugs and biologic agents can supplement treatment with corticosteroids, limiting the potential for side effects.
Although such evidence has come largely from studies on rheumatoid arthritis and other inflammatory disorders, it may prove beneficial in treating ocular inflammation as well. An understanding of the full range of available therapeutic options allows optometrists to better comanage and effectively treat patients with uveitis.
Dr. Wing is an associate professor at the Pennsylvania College of Optometry in
1. McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology: UCLA Community-Based Uveitis Study Group. Am J Ophthalmol 1996 Jan;121(1):35-46.
2. Block-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol 1987 Feb;103(2):234-5.
3. Yanoff M, Duker JS, eds. Chapter 161: General approach to the uveitis patient and treatment strategies. In: Ophthalmology, 2nd ed.
4. The Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data: Results of the first international workshop. Am J Ophthalmol 2005 Sep;140(3):509-16.
5. Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nature Immunology 2006 Apr;7(4):354-59.
6. Durrani
7. Chen L, Gordon LK. Immune mechanisms in uveitis. In: Pleyer U, Mondino B., Eds. Uveitis and Immunological Disorders (Essentials in Ophthalmology).
8. Abbas AK. Chapter 6: Diseases of Immunity. In: Kumar V, Abbas A, Fausto N., Eds. Robbins & Cotran Pathologic Basis of Disease, 7th ed.
9. Chang JH, McCluskey PJ, Wakefield D. Acute anterior uveitis and HLA-B27. Surv Ophthalmol 2005 Jul-Aug;50(4):364-88.
10. Shah KH,
11. Dick AD, Carter DA. Cytokines and immunopathogenesis of intraocular posterior segment inflammation. Ocul Immunol Inflamm 2003 Mar;11(1):17-28.
12. Takase H, Futagami Y, Yoshida T, et al. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Inves Ophthalmol Vis Sci 2006 Apr;47(4):1557-61.
13. Thurau SR, Wildner G. Immunomodulatory therapy in uveitis. In: Pleyer U, Mondino B., Eds. Uveitis and immunological disorders (Essentials in Ophthalmology).
14. Ooi, KGJ, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis Is there a correlation with clinical phenotype? Clin Med Res 2006 Dec;4(4):294-309.
15. Okada AA. Immunomodulatory therapy for ocular inflammatory disease: a basic manual and review of the literature. Ocul Immunol Inflamm 2005 Sep-Oct;13(5):335-51.
16. Kurup SK, Chan CC. Immunotherapeutic approaches in ocular inflammatory diseases. Arch Immunol Ther Exp 2005 Nov-Dec;53(6):484-96.
17. Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum 1994 Apr;37(4):481-94.
18. MedScape Drug Reference. Atropine Opht. Available at: www.medscape.com/druginfo/dosage?drugid=8614&drugname=Atropine+Opht (Accessed June 2007).
19. Siepmann K, Huber M, Stubiger N, et al. Mycophenolate mofetil is a highly effective and safe immunosuppressive agent for the treatment of uveitis: a retrospective analysis of 106 patients. Graefes Arch Clin Exp Ophthalmol 2006 Jul;244(7):788-94.
20. Doycheva D, Deuter C, Stuebiger N, et al. Mycophenolate mofetil in the treatment of uveitis in children. Br J Ophthalmol 2007 Feb;91(2):180-4.
21. Theodossiadis PG, Markomichelakis NN, Sfikakis PP. Tumor necrosis factor antagonists: Preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 2007 Apr-May;27(4):399-413.
22. Foeldvari I, Nielsen S, Kummerle-Deschner J, et al. Tumor necrosis factor- in treatment of juvenile idiopathic arthritis-associated uveitis refractory to second-line agents: Results of a multinational survey. Jour Rheum 2007 May;34(5):1146-50.

