Aniridia is a rare congenital ocular disorder highlighted by a variable hypoplasia or total absence of the iris.1 As a result of mutation or deletion, individuals with aniridia typically lose functionality of the gene that is linked to ocular development.2,3 Patients frequently present with accompanying nystagmus, photophobia and decreased vision. Often associated with concurrent ocular disorders, aniridia can herald more serious systemic complications secondary to the genetic abnormality.
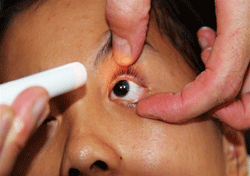
Gross inspection of our 16-year-old patient reveals the presence of bilateral anirida.
The early onset and progressive vision loss seen in these patients typically consists of foveal hypoplasia, followed by late-onset keratopathy, glaucoma and cataracts. Further, the diagnosis of aniridia can be indicative of WAGR syndrome––a rare genetic condition that predisposes individuals to the development of pediatric nephroblastoma (Wilms’ tumor), genitourinary anomalies and mental retardation.
Early diagnosis of aniridia requires a careful ophthalmic evaluation, followed by a prompt referral to rule out WAGR syndrome. Generally, an extensive multidisciplinary approach involving pediatric, imaging, audiology and internal medical subspecialties is indicated.
Available treatment options for aniridia are intended to address associated visual dysfunction and ocular pathology. Intervention varies widely, depending on the condition’s severity. Primary treatment options include corrective and/or tinted lenses as well as low vision aids to enhance visual function.
The treatment of secondary glaucoma and keratitis sicca includes topical, systemic, implantable and surgical modalities. Cataract surgery, while of dubious benefit in mild to moderate cases, is indicated when cataracts are dense and visually obstructive.1,4
Here, we review the case of a young patient who presented with bilateral hereditary aniridia.
History
A 16-year-old Asian female presented to a local vision-screening clinic in a developing nation. She reported a long-standing history of “poor vision, light sensitivity and uncontrollable eye movement.” Despite this, the patient said that she was able to attend school and manage her daily activities with help from family and friends.
Her mother reported that the girl had no significant medical history and was not currently taking any medications. Further, we were unable to determine whether another eye care provider or a general healthcare practitioner had evaluated her previously.
Diagnostic Data
Her uncorrected entering visual acuity measured 20/100 O.U. We documented no improvement upon pinhole testing.
The slit lamp evaluation revealed the presence of immature iris root buds in both eyes. Additionally, we noted the presence of clear corneas, normal anterior chambers, mild congenital cataracts, quiet conjunctivae, and healthy lids and lashes O.U.
Oculomotor function testing was significant for bilateral nystagmus (jerk variety) with mild head tilt (torticollis).
Dilated fundus examination uncovered foveal hypoplasia (no macular reflex), macular hypopigmentation, normal optic nerve (cup-to-disc ratio of 0.4 x 0.4) and no apparent peripheral retinal lesions O.U. Her intraocular pressure measured 20mm Hg O.U.
Diagnosis
The diagnosis in this case is hereditary bilateral aniridia.
Discussion
Aniridia is a congenital panocular disorder highlighted by a variable degree of hypoplasia or total absence of an iris.1-4,5 It is a rare condition, with a reported incidence ranging between one in 40,000 to one in 100,000 in the general population.1,6,7 Usually, it is accompanied by multiple ocular changes that either occur at birth or develop at varying degrees during adolescence. Although there have been some rare reports of aniridia secondary to trauma, most cases are congenital in nature and fall into two categories: hereditary (66%) or sporadic (33%).2
• Hereditary aniridia typically is transmitted in an autosomal dominant manner with high penetrance. The offspring of these patients have a 50% chance of genetic inheritance.1,2
• Sporadic aniridia involves the deletion or mutation of the WT1 gene, which is located on one of the short arms of chromosome 11.1,2,8 The WT1 gene is located with the PAX6 gene, which has an important role in ocular development. Patients with sporadic aniridia often exhibit early development of Wilms’ tumor (a pediatric nephroblastoma), as well as other associated visual and systemic abnormalities. Ninety percent of sporatic aniridia cases have been found to have a compromised copy of the PAX6 gene.1,7
A major diagnostic feature of aniridia is a congenital absence or near absence of the iris, which controls the pupillary size. Foveal hypolasia (absence of a foveal reflex and macular hypo-pigmentation) with reduced vision and an associated nystagmus almost always is present.4,5,9 This infantile variety of nystagmus begins pendular in nature—slow phase movement quickly followed by a quick recovery phase—with the eventual development of the jerk variety between six and 12 months of age.
Patients often will present with a compensatory head tilt (torticollis) in an attempt to find a “null point” that mitigates the severity and amplitude of the involuntary eye movements. Generally, these patients present with vision in the 20/100 to 20/200 O.U. range, and often complain of photophobia.1 Although a majority of cases are documented within a few months of birth, some have manifested later in childhood or even early adolescence. Further, aniridia infrequently presents in infancy as buphthalmos (large corneal diameter and edema).3
Progressive, sight-threatening complications include the gradual development of polar opacities, which are found in up to 85% of aniridics.1 Other lens findings have included tunica vasculosa lentis (fetal vascularization of the anterior lens capsule), and––in rare occasions––lens subluxation.1 More commonly, up to 50% of aniridics develop glaucoma.1,4,8,10
Other late findings include pancorneal vascularization (pannus), opacification and the eventual keratinization of the cornea secondary to chronic keratitis sicca. This occurs as a consequence of stem cell immaturity and eventual failure. Approximately, 10% of patients have exhibited optic nerve hypoplasia.5,9 Refractive errors (myopia, hypermetropia, anisometropia and astigmatism) also are common in these patients.5,9,11
A diagnosis of aniridia typically is not isolated, but rather accompanied by both significant ocular and systemic pathologies. Among the latter findings are various forms of sensory and neurological defects. These include reduced olfaction due to abnormalities of the olfactory bulb, hearing deficits that arise as a result of abnormal inter-hemispheric transfer, and intracranial abnormalities.1 Despite neuroimaging studies that reveal abnormalities of the cerebellum, temporal and occipital lobes and corpus callosum, cognitive development usually is normal; behavioral difficulties and developmental delays are uncommon.1 Any diagnosis of aniridia in a pediatric patient should prompt immediate auditory, otolaryngology and neurologic consults––particularly for cases with demonstrated visual impairment.
WAGR syndrome (and, in turn, Wilms’ tumor, genitourinary anomalies and mental retardation) has been noted in cases of sporadic aniridia that arise from the deletion of the PAX6 and WT1 genes. The PAX6 and WT1 genes are both located on one of the small arms of chromosome 11. It is believed that the accompanying ocular symptoms seen in aniridia cases (e.g., glaucoma, foveal hypoplasia, cataracts, etc.) are due to a compromised PAX6 gene that helps to regulate various ocular functions.1 In these cases, the presence of aniridia is the most consistent symptom.1
Hereditary aniridia presents with familial, intragenic mutations that exhibit a high degree of penetrance.1,5,7 These cases typically don’t present with the morbidity seen in sporadic cases. If Wilms’ tumor is present, a variety of genitourinary anomalies also can be seen, including cryptorchidism, ambiguous genitalia and gonadoblastomas. Other accompanying findings include mental retardation (up to 70% of cases), dysmorphic features, obesity and behavioral abnormalities.1,7,8 In these cases, it is important to assess family history as well as consider genetic counseling as part of the treatment plan.
Wilms’ tumor generally presents by the third birthday, but almost never after the age of eight.1 Approximately 500 new cases are diagnosed per year; 75% occur in normal children, while the remaining are associated with developmental abnormalities.1,7 A majority (95%) are unilateral presentations that respect the abdominal midline and typically metastasize into the lung over time.12 Diagnostic testing to rule out Wilms’ tumor includes neuroimaging (abdominal ultrasound, X-ray or CT scan), as well as a battery of laboratory tests, such as a urinalysis, complete blood count and creatinine clearance.1
In cases where the tumor is diagnosed early and hasn’t metastasized, the majority of patients will respond favorably to treatment (surgical excision, radiation therapy or chemotherapy).
• Management strategies. The eye care provider’s first responsibility is to assure that a diagnosis of aniridia doesn’t involve the presence of WAGR syndrome. Therefore, prompt referral to a multidisciplinary team of pediatricians, oncologists and internal medicine (nephrology) is indicated.
A confirmed WT1 deletion at chromosome 11 requires follow-up renal ultrasound examinations every three months until the age of eight to rule out the development of Wilms’ tumor. Cases of genitourinary abnormalities require monitoring and treatment.1 Baseline audiograms also need to be performed to rule out associated hearing problems.
After a thorough systemic workup, any visual problems (e.g., blurred vision or photophobia) must be addressed using appropriate corrective lenses, low vision aids (e.g., closed-caption television), aniridic contact lenses, or IOLs when the crystalline lenses have been removed due to cataracts and/or photochromic lenses. Measuring visual acuity in infants is a difficult, but necessary, step to determine the extent of iris tissue loss and the presence of both foveal and optic nerve hypoplasia. At the same time, corneal pathology, glaucoma and cataracts also must be ruled out. When accompanied by anisometropia or strabismus, occlusion therapy may be recommended.
If glaucoma is detected, standard medical and surgical treatment is indicated.13 Keratitis sicca, due to a chronically damaged or malformed corneal epithelium, can be addressed using lubrication therapy, punctal occlusion or mycolytics. Limbal stem cell transplantation can be considered in truly recalcitrant cases.2
• Surgical intervention. Cataract extraction surgery is indicated when lens opacities become clinically significant and the prognosis of performing surgery is good. Generally, this is reserved for severe cases, because mild to moderate cataracts often do not respond well to surgical intervention.4 In cases meriting surgery, black diaphragm intraocular lenses have been used to diminish glare and photophobia.1,13
• Genetic considerations. Given the strong evidence of familial link in aniridia, genetic counseling is highly encouraged and must involve the whole family.
Follow-up
Our patient was referred to a multidisciplinary clinic, which included pediatric, imaging and internal medicine consultation, to rule out WAGR syndrome, Wilms’ tumor (nephroblastoma), genitourinary anomalies and mental retardation. Additionally, we recommended a follow-up
ophthalmic evaluation to include low vision testing, UV-blocking sun lenses and genetic counseling at a local eye hospital.
Unfortunately, a follow-up plan never was implemented because the patient was lost to referral.
A diagnosis of aniridia (especially in a child) should prompt an eye care provider to rule out accompanying systemic complications. Although aniridia cases can present simultaneously with various visual and ophthalmic problems, early diagnosis and proper care can help improve the visual and systemic prognosis. Timely referral to a diagnostic medical team, as well as immediate treatment of accompanying systemic complications, can mitigate future problems and enhance a patient’s lifestyle.
Dr. Radoiu is in private practice in Virginia’s Shenandoah Valley.
1. Hingorani M, Hanson I, van Heyningen V. Aniridia. Eur J Hum Genet. 2012 Jun 13;20:1011-7.
2. Lang K. Ophthalmology: A Pocket Textbook Atlas. 2nd ed. New York: Thieme; 2007:208
3. Singh D, Verma A. Aniridia. Medscape. 2008 Jan 17. Available at:
http://emedicine.medscape.com/article/1208379-overview. Accessed November 28, 2012.
4. Lee H, Khan R. Aniridia: current pathology and management. Acta Ophthalmol. 2008 Nov;86(7):708-15.
5. Valenzuela A, Cline RA. Ocular and nonocular findings in patients with aniridia. Can J Opthalmol. 2004 Oct;39(6):632-8.
6. Nelson LB, Speath GL, Nowinski TS, et al. Aniridia. A review. Surv Ophthalmol. 1984 May-June;28(6):621-42.
7. Grønskov, K, Olsen JH, Sand A, et al. Population-based risk estimates of Wilm’s tumor in sporadic aniridia. A comprehensive mutations screening procedure of PAX6 identifies 80% of mutations in aniridia. Hum Genet. 2001 Jun;109(1):11-8.
8. Fishbach BV, Trout KL, Lewis J, et al. WAGR syndrome: a clinical review of 54 cases. Pediatrics. 2005 Oct;116(4):984-8.
9. Hingorani M, Williamson KA, Moore AT, van Heyningen V. Detailed ophthalmologic evaluation of 43 individuals with PAS6 mutations. Invest Ophthalmol Vis Sci. 2009 Jun;50(6):2581-90.
10. Grønskov, K, Rosenberg T, Sand A, Brondum-Nielsen K. Mutational analysis of PAX6; 16 novel mutations including 5 missense mutations with mild aniridia phenotype. Eur J Hum Genet. 1999 Apr;7(3):274-86.
11. McCulley TJ, Mayer K, Dahr SS, et al. Aniridia and optic nerve hypoplasia. Eye (Lond) 2005 Jul;19(7):762-4.
12. Kaneshiro NK. Wilms tumor. ADAM Medical Encyclopedia. Available at:
http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002542/. Accessed November 28, 2012.
13. Khaw PT. Aniridia. J Glaucoma. 2002 Apr;11(2):164-8.

