For the most part, optometrists opt to initiate glaucoma treatment using topical medications. In our practice, we strive to treat each patient to the fullest extent of our licensure. But what happens when a patient’s IOP cannot be adequately controlled with medical intervention alone? That’s when we refer to a glaucoma specialist. Sometimes, that means the patient will undergo glaucoma surgery. Fortunately for them, surgical options are less invasive and safer than ever before. Laser and surgical procedures such as laser trabeculoplasty, minimally invasive glaucoma surgery (MIGS) or incisional glaucoma surgery may be just the solution for these patients. But just because they’re seeking treatment in an MD’s office, that doesn’t mean our job is done.
This article provides an overview of these modern procedures and reviews the OD’s role in managing patients who have undergone them.
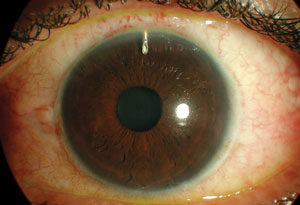 | |
| The Express shunt under a scleral flap, post-op day one. The bleb is elevated but fairly diffuse, the chamber is deep and there is little inflammation. This is one of several available glaucoma drainage implants. |
Drop Compliance
Medication compliance is an elusive part of glaucoma management. We have all encountered patients who insist that they comply with their eye drops, yet have not filled the order since treatment was initiated.
That lack of adherence, a study shows, turns out to be one of the primary causes of IOP fluctuation.1 And IOP fluctuation is associated with an increased risk of visual field progression, particularly in patients with a lower initial mean IOP.2-4
It is imperative for patients to understand the need for strict compliance with therapy to prevent fluctuations that hasten the progression of their disease.
Although several topical glaucoma drops may significantly lower eye pressure, some patients may need additional methods of intervention to reach a suitable IOP. The Advanced Glaucoma Intervention Study (AGIS): 7 shows a lower IOP is associated with a reduced progression of visual field defects.5 Furthermore, those with IOPs less than 18mm Hg showed minimal visual field worsening in a six-year period.5
These patients may be treated with procedures such as trabeculectomy or receive one of several available glaucoma drainage implants (GDI).
Trabeculectomy
Trabeculectomy is a commonly performed, non-laser glaucoma surgery in which a fistula connecting the anterior chamber and the subconjunctival space is created to provide an alternative pathway of aqueous outflow.6-8 Success depends upon establishing proper filtration and ensuring the patency of the fistula.9
Antimetabolites
Antimetabolites are chemical agents typically used during incisional glaucoma surgery to reduce conjunctival scarring and the likelihood of procedure failure. The two antimetabolites commonly used today are 5-fluorouracil (5-FU) and mitomycin-C (MMC).
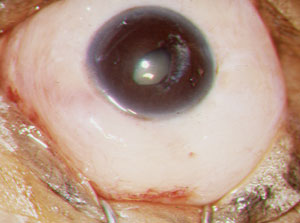 | |
| A completed trabeculectomy with a diffuse, 360-degree filtration bleb. |
5-FU inhibits fibroblast proliferation whereas MMC causes crosslinking of DNA and may also inhibit RNA and protein synthesis. Both antimetabolites work well, but must be used with caution, as corneal toxicity and wound leaks are common. In particular, MMC is historically considered more potent because the complications are more severe.10
Late Postoperative Trabeculectomy Complications
When evaluating a patient with a trabeculectomy, doctors should examine certain key features. Signs of a healthy, well-functioning bleb include the presence of multiple intraepithelial microcysts (which can be viewed easily with fluorescein sodium), minimal vascularity, decreased height, thick walls and the absence of corkscrew vessels. It is also good practice to use a fluorescein strip to evaluate for the presence of a bleb leak as evidenced by a positive Seidel test. Since aqueous does not take up fluorescein dye, a bleb leak will appear as dark flow surrounded by normal-appearing dye on the corneal surface.11
Late trabeculectomy complications are often attributed to changes in the bleb’s structure. The most common of these include chronic hypotony, bleb leaks and blebitis.12
Chronic hypotony is defined as an intraocular pressure of less than 5mm Hg for at least a three-month period. Non-surgical treatment options include the use of a soft contact lens, cryotherapy to reduce bleb size, autologous blood injection with or without compression sutures, and applying an argon laser to the bleb. However, surgical revision is the most successful modality to elevate intraocular pressure. Rarely, choroidal effusion or suprachoroidal hemorrhage may result from longstanding hypotony.12
Trabeculectomy Steps
1. Khaimi M, Reyes M. Filtering Surgery in the Management of Glaucoma. In: Kahook MY SJED, ed. Chandler and Grant’s Glaucoma. 5th ed. Thorofare, NJ:SLACK, Inc.;2013. |
The use of antimetabolites is associated with thin-walled blebs and bleb leaks.10,11 Aqueous suppressants, broad-spectrum antibiotics, patching and soft contact lenses may be used to close the leak. Other treatment options include cyanoacrylate glue, fibrin tissue glue, injection of autologous blood and surgical intervention.12
Thin-walled blebs are also at a higher risk for infection and blebitis. The infected bleb will typically exhibit a local conjunctival hyperemia and a milky-white appearance. In addition to thin walls, the presence of myopia, releasable sutures, respiratory infections, inferior limbus blebs, unguarded filtration surgery and diabetes increase the risk for bleb infection. Blebitis may also be associated with bleb leak, hypopyon, vitreous reaction, and may even develop into an endophthalmitis.12
Symptomatic blebs provide varying degrees of discomfort and often result in superficial punctate keratopathy, dellen formation and ocular surface irregularities. The recommended treatment to alleviate irritation and discomfort is copious artificial tears and lubrication. If symptoms persist after frequent use of lubrication, compression sutures may be placed to compress the bleb to the sclera.
Trabeculectomies remain the initial incisional glaucoma procedure for many surgeons; however, the glaucoma drainage implants have grown in popularity recently due to their successful use in secondary glaucoma and failed blebs.
Drainage Implants
GDIs
Glaucoma drainage implants (GDI) are devices constructed of a tube that shunts aqueous humor from the anterior chamber to an encapsulated fibrous plate located at the equatorial region of the globe. The aqueous fluid is absorbed by the periocular capillaries and lymphatic system of the subconjunctival space. Although the basic concepts of all GDIs are the same, the implants differ with respect to shape, material, size and presence of a valve.13 Despite all of the design differences, a systematic literature review found that all glaucoma drainage implants effectively lowered the preoperative IOP by at least 50%.14
MIGS is growing in popularity because it is effective and less disruptive to the native ocular tissue than prior surgical interventions. Procedures such as Trabectome and shunt or stent implantation are useful options for mild to moderate glaucoma, but may not be a suitable for patients with various forms of secondary, advanced or refractory glaucoma.1-4 1. AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS):7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. Oct 2000;130(4):429-40. 2. Minckler D, Mosaed S, Dustin L. Trabectome Study Group. Trabectome (trabeculectomy-internal approach): additional experience and extended follow-up. Trans Am Ophthalmol Soc. 2008;106:149-59. 3. Craven E, Katz L, Wells J, Giamporcaro J. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–45. 4. Sameulson J, Katz L, Wells J, et al., US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–67. |
Implant Size
A study comparing single and double-plated Molteno implants proposed that larger end plates provide a larger IOP reduction.15 However, this data has not been thoroughly substantiated. End plate size does appear to have some effect on the IOP, although this may be true only to an extent. The ideal size of the end plate is not known, and research shows conflicting data.16 A recent study comparing Baerveldt Glaucoma Implants (BGI) with end plate sizes of 250mm2 and 350mm2 concluded that there was no difference in surgical success, IOP, VA or topical medications used throughout a three-year period.17
Valved vs. Non-Valved
The design of a GDI may include the presence of a valve, which regulates aqueous flow through the tube to the end plate. This idea may seem contradictory to the intended purpose of a GDI; however, research shows the function to be an asset in the postoperative period. A valved GDI helps to restrict further aqueous outflow if the IOP drops too low. Furthermore, valved implants also appear to reduce the risk of postoperative hypotony.13
The drainage implants most commonly used are the Ahmed Glaucoma Valve (New World Medical) and the Baerveldt Glaucoma Implant (Advanced Medical Optics). Other implants that are also available include the Krupin slit valve (Hood Laboratories) and the Molteno implant (Molteno Ophthalmic Limited).13
Late Post-op GDI Complications
Glaucoma drainage implants have several complications not typically seen with trabeculectomies. These complications include tube and plate migration, bleb encapsulation, diplopia and device exposure.13
Tube and plate migration can occur as a late postoperative complication for glaucoma drainage implants. Tube extenders are used to lengthen retracted tubes. A migrating plate occurs less commonly than tube migration, but can be corrected by resuturing the GDI.
| iStent Implant By Barbara Fluder, OD The iStent (Glaukos) is essentially a small snorkel comprised of heparin-coated titanium implanted nasally into Schlemm’s canal during cataract surgery to improve aqueous outflow by creating a permanent conduit to the trabecular meshwork. Contraindications: Primary or secondary angle closure, neovascular glaucoma, thyroid eye disease, Sturge-Weber syndrome or any other condition that may cause elevated episcleral venous pressure. Preoperative care: According to the company’s website, gonioscopy should be performed before surgery to rule out peripheral anterior synechiae, rubeosis and any angle abnormalities or conditions that would occlude sufficient visualization of the angle that could lead to improper placement of the stent. Postoperative care: Cataract recovery drops as well as the patient’s prescribed glaucoma medications. |
Encapsulation of the bleb may result in a failure to control IOP after glaucoma drainage implant surgery. This complication is comparable to an encapsulated bleb that develops after trabeculectomy. Both are generally treated with topical antihypertensive medications.
Persistent restrictive strabismus may occur after a GDI because of scarring between the rectus or oblique muscles and the implant, or due to a crowding effect from a large bleb with limitation of extraocular motility. The most common treatment for diplopia secondary to drainage devices is prismatic correction, but muscle surgery or removal of the drainage implant may be necessary to negate the diplopia.
Partial or complete exposure of the GDI is one of the more frequent and challenging complications of glaucoma surgery. Exposure of the device is difficult to treat and may require removal of the device.
The Studies
Tube vs. Trabeculectomy Study
The Tube vs. Trabeculectomy (TVT) study was a multicenter randomized clinical trial comparing the safety and efficacy of tube shunt surgery with the Baerveldt glaucoma implant (BGI model 101-350) and trabeculectomy with mitomycin C in patients with previous cataract surgery, filtering surgery or both. Several interesting features can be teased out from this study through five years of follow up:21
- Tube shunt surgery with a GDI had a higher success rate than trabeculectomy with MMC during the first five years of the study.
- Long-term IOP was slightly lower with trabeculectomy over tube shunt.
- Early postoperative complications occurred more frequently after trabeculectomy with MMC than after GDI surgery.
- The rates of late postoperative complications and reoperation for complications were similar with both surgical procedures during five years of follow up.
- The rate of vision loss was similar between both groups.
Overall, the results demonstrated no clear superiority of one glaucoma operation over the other. Both surgical procedures are viable options for patients with previous cataract extraction, failed trabeculectomy, or both. The TVT Study does support the practice pattern shift of a trend towards the use of more GDIs in this population.18
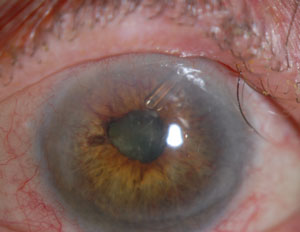 | |
| A patient with neovascular glaucoma after central retinal vein occlusion, with an Ahmed Glaucoma Valve. Image: Peter A. Netland, MD, PhD. |
GDI Studies
The Ahmed Baerveldt Comparison Study (ABC) compared the safety and efficacy rate of IOP lowering after implantation of the Ahmed Glaucoma Valve (AGV model FP7) and Baerveldt Glaucoma Implant (BGI model 101-350) devices in 267 refractory glaucoma patients. Both AGV and BGI devices reduced IOP and medication usage through five years. Both groups in the ABC study observed a greater than 50% reduction in IOP and similar rate of treatment failure, albeit for different reasons. Most AGV failures were attributed to high IOP, while most BGI failures were a result of low IOP or postoperative complications. One notable comparison is the finding that the BGI group had slightly lower IOPs at five years while taking considerably fewer postoperative glaucoma medications.19,20
The Ahmed Versus Baerveldt (AVB) study compared the long-term effectiveness of the AGV with the BGI in patients with refractory or high-risk glaucoma. The 238 patients enrolled in this study had uncontrolled IOP at the time of surgery, and many had a previously failed trabeculectomy. The AVB study found both GDIs to be effective at reducing intraocular pressure and the dependence on topical glaucoma medications. The Baerveldt group had a lower failure rate, but experienced more hypotony-related vision threatening complications through three years of follow up.20
The ABC and AVB studies have found both the Ahmed Glaucoma Valve and Baerveldt Glaucoma Implant to be effective surgical options for the management of refractory glaucoma. The studies also determined that AGVs were more likely to fail due to high intraocular pressure postoperatively whereas BGIs failed due to a low pressure and associated complications. These results can be partly explained by the presence of a flow-restricting valve on the AGV implant and the valveless design of the BGI. A GDI with a valve is advantageous for early postoperative IOP control and reduced hypotony risk.18,21
GDI Steps
1. Salim S, Kahook M. Aqueous Shunting Procedures. In: Kahook MY SJED, ed. Chander and Grant’s Glaucoma. 5th ed. Thorofare NJ: Slack Inc; 2013. |
Your patients may be good candidates for incisional glaucoma surgery if they have advanced glaucoma, a form of secondary glaucoma, demonstrate significant IOP fluctuation or cannot tolerate medical treatment. Although the patient and ophthalmologist will ultimately make the surgical decision, it is important for optometrists to understand the basic concepts of incisional glaucoma surgery and potential postoperative complications. The initial glaucoma procedure for many ophthalmologists is a trabeculectomy; however, results from the TVT study show that the IOP reduction and overall surgical success is generally equal between a trabeculectomy and a glaucoma drainage implant. As such, the use of glaucoma drainage implants has increased in recent years. Patients with failed trabeculectomies or those with high risk may benefit from aqueous drainage devices.
Dr. Zimbalist and Dr. Gentry practice at the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Mo.
1. Gray T, Fenerty C, Harper R, et al. Individualised patient care as an adjunct to standard care for promoting adherence to ocular hypotensive therapy: an exploratory randomised controlled trial. Eye (Lond). 2012 Mar;26(3):407-17.2. Caprioli J, Coleman A. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115(7):1123-9.
3. Hong S, Seong G, Hong Y. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol. Aug 2007;125(8):1010-3.
4. Komori S, Ishida K, Yamamoto T. Results of long-term monitoring of normal-tension glaucoma patients receiving medical therapy: results of an 18-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2014 December;252(12):1963-70.
5. AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS):7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. Oct 2000;130(4):429-40.
6. Healthwise Staff. Trabeculectomy (Filtration Surgery) for Glaucoma. WebMD. February 28, 2012. Available at: http://www.webmd.com/eye-health/trabeculectomy-filtration-surgery-for-glaucoma. Accessed December 11, 2014.
7. Sugar H. Some recent advances in the surgery of glaucoma. Am J Ophthalmol. 1962;54:917-29.
8. Cairns J. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968;66:673-9.
9. Pia Uyloan de Guzman M. Trabeculectomy. eMedicine. Jan 22, 2014. Available at: http://emedicine.medscape.com/article/1844332-overview. Accessed Dec 08, 2014.
10. De Fendi L, Arruda G, Scott I, Paula J. Mitomycin C versus 5-fluorouracil as an adjunctive treatment for trabeculectomy: a meta-analysis of randomized clinical trials. Clin Experiment Ophthalmol. 2013 Nov;41(8):798-806.
11. Picht G, Grehn F. Classification of filtering blebs in trabeculectomy: biomicroscopy and functionality. Curr Opin Ophthalmol. 1998;9(2):2-8.
12. Vijaya L, Manish P, Ronnie G, Shantha B. Management of complications in glaucoma surgery. Indian J Ophthalmol. 2011;59:S131-40.
13. Schwartz K, Lee R, Gedde S. Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol. 2006;17:181-89.
14. Hong C, Arosemena A, Zurakowski D, Ayyala R. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. Jan-Feb 2005;50(1):48-60.
15. Heuer D, Lloyd M, Abrams D, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. Oct 1992;99(10):1512-9.
16. Freedman J. Preoperative Evaluation of Patients Undergoing Drainage Implant Surgery. In: Shaarawy TM SMHRCJ, ed. Glaucoma. 2nd ed. China: Elsevier Limited; 2015.
17. Allan E, Khaimi M, Jones J, Ding K, Skuta G. Long-term Efficacy of the Baerveldt 250 mm(2) Compared with the Baerveldt 350 mm(2) Implant. Ophthalmology. Mar 2015;122(3):486-93.
18. Budenz D, Barton K, Gedde S, et al. Ahmed Baerveldt Comparison Study Group. Five-year treatment outcomes in the Ahmed Baerveldt Comparison Study. Ophthalmology. 2015;122(2):308-16.
19. Barton K, Feuer W, Budenz D, et al. Ahmed Baerveldt Comparison Study Group. Three-year treatment outcomes in the Ahmed Baerveldt Comparison Study. Ophthalmology. 2013;121(8):1547-57.
20. Christakis P, Tsai J, Kalenak J, et al. The Ahmed Versus Baerveldt Study: three-year treatment outcomes. Ophthalmology. 2013;120(11):2232-40.
21. Gedde S, Schiffman J, Feuer W, et al. Tube Versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) Study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789-803.


