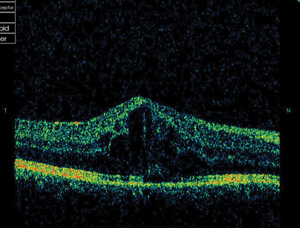
 |
| Adalimumab fared better than infliximab in this study for treatment of noninfectious uveitis. Photo: Michael Trottini, OD, and Candice Tolud, OD. Click image to enlarge. |
Noninfectious uveitis is an immune-mediated response commonly associated with systemic diseases or non-systemic inflammatory conditions, or it may be idiopathic. While corticosteroids are the mainstay of treatment, steroid-sparing therapies such as anti–tumor necrosis factor-α (anti-TNFα) biologics and systemic immunomodulators allow for steroid tapering and mitigation of long-term side effects. Humira (adalimumab [ADA], AbbVie) is the first and only FDA-approved anti-TNFα, and Remicade (infliximab [IFX], Janssen Biotech) has also been explored as an off-label treatment.
Researchers recently found low quality evidence suggesting that ADA treatment may result in lower central macular thickness (CMT), higher final best-corrected visual acuity (BCVA) and lower rates of adverse event occurrence and associated discontinuation compared with IFX.
The team conducted a literature review of 5,836 studies, 12 of which met inclusion criteria and were included in the meta-analysis. The reported final logMAR BCVA in the studies was significantly better in ADA compared with IFX. No significant differences between treatment with ADA and IFX were found regarding corticosteroid-sparing effect. There was evidence to suggest that ADA may result in a better CMT compared with IFX at last follow-up. The average CMT was 252.6±37.1μm and 259.0±20.1μm for IFX and ADA, respectively.
Pooled analysis demonstrated no significant difference in the remission proportion between ADA and IFX. Relapse rates aggregated from 11 studies showed no significant difference between treatment with IFX or ADA. The comparative mean relapse rates were 0.071 and 0.104 events per 100 patient-years for ADA and IFX, respectively. Risk of bias assessment showed that all outcomes were of “low quality” due to the presence of confounding bias in observational studies, with the exception of final logMAR BCVA, which was “very low quality” due to the heterogeneity in outcome measures.
Regarding safety outcomes, pooled analysis showed lower rates of discontinuation proportion due to adverse events in ADA compared with IFX. Patients receiving IFX had significantly more AEs compared with ADA (20.9% IFX vs. 13.7% ADA). Patients receiving ADA showed significantly lower rates of acute reactions (e.g., injection site reaction, allergic reaction, skin rash) and non-specific reactions (e.g., headache, myalgia, arthralgia, fatigue) compared with IFX.
“These findings have important implications” for guiding eye care providers in selecting the most appropriate management strategy, the study authors concluded. “Future studies comparing IFX and ADA for specific systemic diseases or types of [noninfectious uveitis] and reporting standardized outcomes for efficacy and safety during consistent and longer follow-up intervals would help to further define the differences between these two agents and guide ophthalmologists in selecting the most appropriate management strategy.”
Tai F, Far PM, Pechlivanoglou P, et al. Efficacy and safety of adalimumab and infliximab for non-infectious uveitis. Ophthalmology. October 8, 2021. [Epub ahead of print]. |


