A
weekly e-journal by Art Epstein, OD, FAAO
|
|
Volume 15, Number 6 |
Monday, February 9, 2015 |
Off the Cuff: The Point of No Return
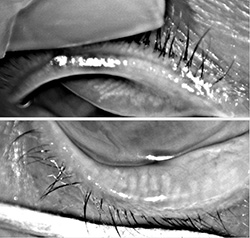
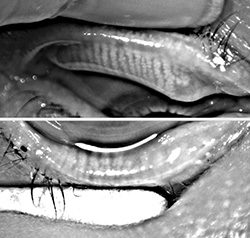
For those deep into dry eye and ocular surface disease, recognition of the role that functioning meibomian glands play in maintaining a stable tear structure and reducing evaporation came as an epiphany. As far as I am concerned, MGD is the root cause of nearly all dry eye disease.
Today, most experts believe that if left untreated, obstructed meibomian glands will down-regulate, cease functioning and ultimately
wither and die. But when does a non-functioning meibomian gland become truly dead? That question has been a point of debate.
Some believe that once non-functional, the meibomian gland is gone forever, while others—myself included—believe that
glands can recover if treated before they reach the point of no return.
|
|||||||||
|
|
|||||||||

|
||
| Effect of Watching 3-D Television on Refractive Error in Children | ||||
|
Sixty healthy volunteers, aged six to 12 years, without any ocular abnormalities other than refractive error, were recruited for this study to
investigate the effect of watching 3-D television on refractive error in children. They watched 3-D TV for 50 minutes at a viewing distance of
2.8 meters. The image disparity of the 3-D contents was from -1° to 1°. Refractive errors were measured both before and immediately after
watching TV and were rechecked after a 10-minute rest period. The refractive errors before and after watching TV were compared. The amount of
refractive change was also compared between myopes and controls. The refractive error of the participants who showed a myopic shift immediately
after watching TV were compared across each time point to assure that the myopic shift persisted after a 10-minute rest.
The mean age of the participants was 9.23 ± 1.75 years. The baseline manifest refractive error was -1.70 ± 1.79 (-5.50 to +1.25) diopters. The refractive errors immediately after watching and after a 10-minute rest were -1.75 ± 1.85 and -1.69 ± 1.80 diopters, respectively, which were not different from the baseline values. Myopic participants (34 participants), whose spherical equivalent was worse than -0.75 diopters, also did not show any significant refractive change after watching 3-D TV. A myopic shift was observed in 31 participants with a mean score of 0.29 ± 0.23 diopters, which resolved after a 10-minute rest. Watching properly made 3-D content on a 3-D TV for 50 minutes with a 10-minute intermission at more than 2.8 meters of viewing distance did not affect the refractive error of children. |
||||
|
SOURCE: Kim SH, Suh YW, Choi YM, et al. Effect of watching 3-dimensional television on refractive error in children. Korean J Ophthalmol. 2015;29(1):53–7. |
||||

|
||
| Cocaine-Induced Ocular Surface Damage via Corneal Sensitivity Impairment | ||||
|
Cocaine abuse may cause severe ischemic and necrotic tissue damage in several organs, including the eye. However,
the cornea is an avascular tissue relying on sensitive nerves for its trophic support, and the pathogenesis of cocaine-induced corneal lesions
is unclear. In this study, we aimed to investigate whether corneal sensitivity, ocular surface and tear function are damaged by habitual cocaine
snorting. Ocular examination, corneal sensitivity and tear function testing were carried out in 48 cocaine addicts, and in 22 heroin addicts and
30 drug-free age/sex-matched individuals who served as controls. We also performed corneal confocal microscopy, conjunctival impression cytology
and tear sample collection to evaluate corneal and conjunctival morphology, as well as the presence of cocaine in tears. Statistical analysis was
performed to compare groups and to correlate clinical findings with anamnestic data on cocaine use.
We observed decreased corneal sensitivity in 26 cocaine addicts, and neurotrophic keratitis in six of them, with corneal damage, absence of symptoms, reduced tear production and prolonged inter-blink time. No significant changes in ocular surface parameters including corneal sensitivity were observed in heroin addicts. The major risk factors for developing cocaine-induced neurotrophic keratitis appeared to be duration and frequency of drug abuse. A complete ophthalmic evaluation including corneal sensitivity testing should be planned for an optimal management of cocaine addicts, even in the absence of ocular symptoms, to reduce the risk of corneal lesions and consequent vision impairment. Sensory nerve damage should also be evaluated in cocaine-induced lesions of other organs. |
||||
|
SOURCE: Mantelli F, Lambiase A, Sacchetti M, et al. Cocaine snorting may induce ocular surface damage through corneal sensitivity impairment. Graefes Arch Clin Exp Ophthalmol. 2015; Feb 3. [Epub ahead of print]. |
||||

|
||
| Evaluation of Corneal Endothelium in Adolescents With Juvenile Glaucoma | ||||
|
This study evaluated the endothelial cell density (ECD) and central corneal thickness (CCT) in adolescents with
juvenile open-angle glaucoma (JOAG) and ocular hypertension (OH) and to investigate the influence of topical anti-glaucoma medications on ECD and
CCT in adolescents with JOAG.
ECD and CCT were investigated in 66 eyes of 33 adolescents with JOAG. Depending on the topical treatment the eyes were classified into four groups: (1) topical carbonic anhydrase inhibitor; (2) prostaglandin analogs; (3) beta-blocker; and (4) CAI-beta-blocker combination. ECD and CCT were also checked in 24 adolescents with OH and in control group (33 persons). ECD was significantly lower in eyes with JOAG (2639.5 cells/mm²) compared with ECD in eyes with OH (2924.5 cells/mm²) and in control group (2955.5 cells/mm²). CCT was 0.554 mm in eyes with JOAG, 0.55 mm in eyes with OH, and 0.544 mm in control group. ECD in patients with JOAG was 2730 cells/mm² (group one), 2773.5 cells/mm² (group two), 2539.5 cells/mm² (group three), and 2551 cells/mm² (group four). CCT was 0.556 mm in group one, 0.558 mm in group two, 0.532 mm in group three and 0.544 mm in group four. Our findings indicate that JOAG and OH did not affect CCT, but JOAG has influence on ECD in adolescents. There were no significant differences between ECD and CCT of eyes treated with different kinds of anti-glaucoma medications. |
||||
|
SOURCE: Urban B, Bakunowicz-Lazarczyk A, Michalczuk M, Krętowska M. Evaluation of corneal endothelium in adolescents with juvenile glaucoma. J Ophthalmol. 2015;2015:895428. Epub 2015 Jan 6. |
||||
| News & Notes | ||
| ALCON RECEIVES FDA APPROVAL OF PAZEO SOLUTION FOR RELIEF OF OCULAR ALLERGY ITCH. The FDA has approved Alcon's Pazeo (olopatadine hydrochloride ophthalmic solution) 0.7% for the treatment of ocular itching associated with allergic conjunctivitis. Pazeo solution is dosed one drop daily and was approved with efficacy data at 24 hours post dose. Results from two conjunctival allergen challenge clinical studies showed that Pazeo 0.7% demonstrated statistically significantly improved relief of ocular itching associated with allergic conjunctivitis at 24 hours post treatment compared to olopatadine HCl 0.2% (Pataday, Alcon). Pazeo solution is anticipated to be available by prescription in the United States in March 2015, followed by Latin American and Asian markets through 2017. Find out more at www.alcon.com. | ||
|
|
||
| FDA CLEARS CYCLO G6 LASER SYSTEM FOR TREATMENT OF MULTIPLE STAGES OF GLAUCOMA. Iridex Corp. has received clearance from the FDA to market its first laser system designed solely for use in treating glaucoma and its symptoms. Specifically, the company was granted 510(k) clearance for the Iridex Cyclo G6 Laser System (with delivery devices). The system is dedicated specifically to treat patients diagnosed with a range of glaucoma disease states and features the company's proprietary MicroPulse tissue-sparing technology and a family of single-use probes that connect to an intuitive, user-friendly laser console. According to Iridex, the Cyclo G6 Laser System will initially be sold with two disposable delivery probes, the MicroPulse P3 probe and the G-Probe. For additional information, visit www.iridex.com. | ||
|
|
||
| POSITIVE CLINICAL STUDY FINDINGS REPORTED FOR BIOLIGHT'S TEARX DIAGNOSTIC PARAMETERS FOR DRY-EYE SYNDROME. BioLight Life Sciences Investments Ltd. recently announced the completion of a U.S. prospective clinical study conducted by Ora Inc. that compared widely used benchmark tests for dry eye (e.g., Schirmer's test, tear film break-up time, patient questionnaires) with its TeaRx diagnostic parameters that test components of tear film. Positive statistical correlations were identified between TeaRx diagnostic parameters and widely used benchmark tests for dry-eye syndrome. BioLight plans to initiate a second clinical trial to assess the effectiveness of the tests in tears of healthy subjects as well as patients with severe dry-eye syndrome. The trial results are expected in Q2 2015 and should enable the company to specifically define the combination of parameters to be part of a commercially available diagnostic kit and then initiate the regulatory processes for the kit. Assuming the completion of a successful development and regulatory process, BioLight expects to obtain regulatory approvals and launch the multi-parameter test kit in 2016. The TeaRx diagnostic tests are developed by DiagnosTear Ltd., one of BioLight’s subsidiaries. | ||
|
|
||
| THE POWER PRACTICE LAUNCHES INZUZO ANALYTICAL SOFTWARE. As a result of a strategic alliance with Glimpse, the Power Practice is launching Inzuzo, an analytical software exclusively available to Power Practice clients. The software allows a doctor’s Power Practice support team to instantly have a real time, detailed and accurate window into the practice’s operations from which to counsel and coach on ways to improve the practice. Learn more about the Power Practice and/or Glimpse. | ||
|
|
||
| BLEPHEX LAUNCHES FIRST CERTIFIED LID HYGIENIST PROGRAM. BlephEx LLC plans to offer concentrated courses at a variety of locales around the nation in conjunction with major ophthalmic meeting to provide education and practical training in the use of BlephEx, a device that delivers microblepharoexfoliation to remove excess oil, debris and inflammation-promoting biofilm from the eyelid margins. The programs are designed not only for doctors who desire more thorough hands-on instruction for improved techniques, but more specifically, for ophthalmic technicians who wish to become certified in the patient management and treatment protocols necessary to employ BlephEx technology. For additional information and upcoming course locations, click here. | ||
|
|
||
| HELMSLEY CHARITABLE TRUST AWARDS ALLEGRO OPHTHALMICS $2 MILLION. Allegro Ophthalmics LLC has been awarded a $2 million grant from the Type 1 Diabetes Program of The Leona M. and Harry B. Helmsley Charitable Trust. This is the second grant from the Helmsley Charitable Trust to Allegro, and will support the company’s recently commenced Phase II study of Luminate (ALG-1001) in patients with diabetic macular edema. Luminate, a first-in-class Integrin Peptide Therapy, treats vitreoretinal diseases by targeting integrin receptors involved in cell signaling and regulation, and in the construction of new and aberrant blood vessels. It is not approved by the FDA for commercial sale in the United States. The study, which began in October, is evaluating the safety and efficacy of intravitreal injections of Luminate compared to the current standard of care for patients with DME. The total planned enrollment for the six-month, randomized, double-masked, multi-centered trial is 150 patients. Get more details at www.allegroeye.com. | ||
|
|
||
|
|
Optometric Physician™ (OP) newsletter is owned and published by Dr. Arthur Epstein. It is distributed by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
HOW TO ADVERTISE
|