| |
Volume 11, Number 1 |
March
2015 |
|
|
Inside
This Issue
|
|
|
|
|
|
This e-newsletter is provided free to doctors through industry support from |

|
 |
| |
FROM
THE DESK OF THE EDITOR
Someone recently asked me about lecturing. They actually asked
many insightful questions, most of which I could not sufficiently answer. Essentially, it boiled down to why do we
do things like lecture, or write articles, or get involved in things other than just seeing patients? Is it because
we are egomaniacs and like to see our name out there? Maybe. Is it because I love to travel and sit on an plane next
to strangers who think I am interested in their life story? Definitely not!
LIVE
POLL |
Should recommendations for ocular nutritional supplements be based on genetics or do you think it doesn't matter?
|
I think I do it out of a sense of giving back to the profession.
In my case, I am not really interested in politics, but I do like to talk, and teach. Therefore, lecturing seemed to
be something that I had the talent and desire to do. In optometry, I think it is all about finding your niche, deciding
what it is in the profession that you like to do, and have a proclivity for. Hopefully, this something is something
you can make a living at, and benefits the profession as a whole. If so, it is a win-win and you need to capitalize on that talent.
I am also happy to announce that the ORS and Review of Optometry will be partnering again to offer 12 hours of CE in
sunny Anaheim, CA December 4th and 5th. More details will be coming soon, but save those dates if you would like to join us
and learn about the latest research, advancements and treatments in vitreoretinal disorders. Topics will appeal to both
general optometrists looking to improve their understanding of retinal disease and also those with a particular
interest in retina.
For more information and registration, click
here.
Hope to see you there!
Also, please see the first message from our new ORS President Brad Sutton. Brad is the
Clinic Director for Indiana University's Indianapolis Eye Care Center, and is on faculty at IU.
He has been quite active in the ORS filling many roles for us, including treasurer most
recently. I have no doubt that Brad will be an excellent president, even though he has large
Italian, designer shoes to fill from our previous president, Joe Pizzimenti!
Steven Ferrucci, O.D., F.A.A.O.
Editor in Chief
 |
PRESIDENT'S MESSAGE
Brad Sutton, O.D., F.A.A.O.
As I begin my two-year term as the President of the ORS, I can't help but reflect on the time
that I have spent as an ORS Fellow. Serving as the ORS Secretary during Joe Pizzimenti's
recently concluded presidency proved to be an excellent segue to the President's position.
DECEMBER 2014
POLL RESULTS
 |
Joe's leadership and passion for the ORS were remarkable. Joe, and each of the President's before
him (Bill Jones, Jerry Sherman, and Larry Alexander), all brought something special to the ORS.
I truly hope to be able to do the same over the next two years.
The ORS has grown significantly since a handful of visionary individuals with a passion for the
retina became its founding Fellows in 2003. We now are fortunate enough to have nearly forty
Fellows, with more applications arriving regularly. Over the years, my favorite thing about fellowship
in the ORS has been developing wonderful relationships and comradery with other Fellows. We bounce
ideas off of one another. We regularly share clinical images and cases, both to seek advice from
others and merely to share our passion for what we do every day.
Fellowship in the ORS is truly
a rewarding endeavor, and I encourage anyone who thinks that they may have an interest to
consider applying. The criteria for fellowship are outlined on our web page
(
www.optometricretinasociety.org),
as are instructions for the application process. We are a welcoming group, so give us a look!
Brad
ORS President

YOU
MAKE THE DIAGNOSIS
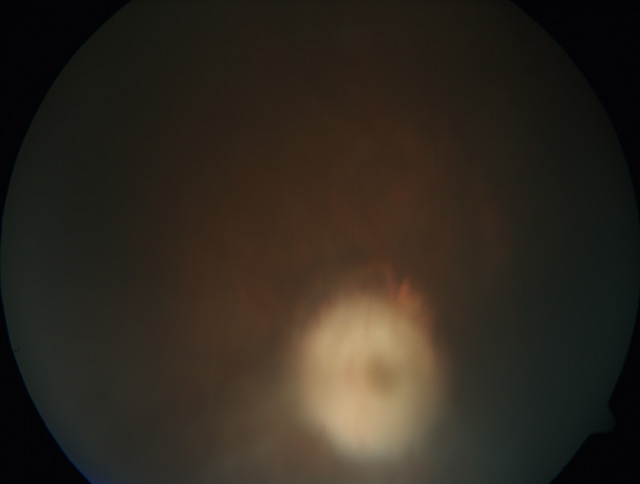 |
|
Figure 1 |
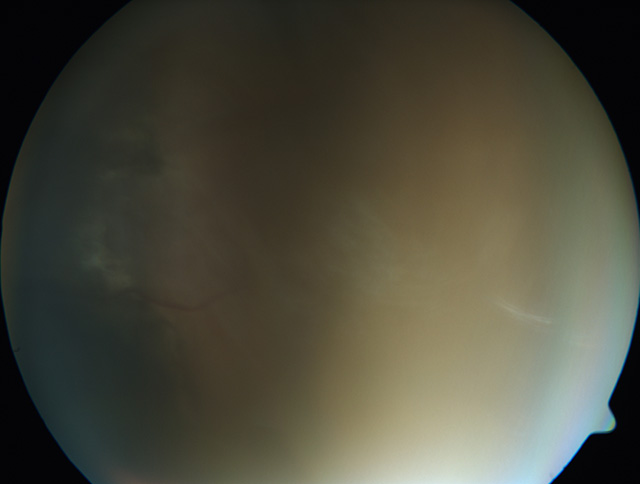 |
|
Figure 2 |
Answer appears later in newsletter.

|
CLINICAL
PEARLS
Posterior Vitreous Detachment: A Few Pearls
Given the new guidelines (AAOphth Oct 2014 Retina/Vitreous PPP Panel ) and the fact that 8-26%
of patients presenting with an acute Posterior Vitreous Detachment (PVD) may have a concomitant
retinal break at the time of the initial presentation, the importance of a careful peripheral
retinal evaluation at the initial exam is paramount. This also suggests that it may be best to follow
up an acute PVD earlier rather later. Further, the chance of developing a retinal break after the
initial presentation of an acute PVD is <5%, so continued care is warranted.
By Diana Shectman, O.D., F.A.A.O.
ORS Fellow
Speaking as a PVD "victim" I tell patients that the symptoms subside significantly during the first week
and dissipate during the next 6 weeks. But, the floaters (OD) and the occasional flash (OS) never
completely disappear. And YES, I did get a dilated evaluation on both occasions.
By Leo Semes, O.D., F.A.A.O.
ORS Founding Fellow
As a patient, flashes are far easier to detect in complete darkness than under room lit conditions.
That's how I detected my first retinal tear.
I tell patients w acute onset PVDs w/o detected tears to monitor themselves.
Any change warrants immediate attention.
By Jerome Sherman, O.D., F.A.A.O.
ORS Founding Fellow
Looking For Diabetic Macula Edema
For Detecting Diabetic Macular Edema – Don't Just Look At B-scan
By Mark Dunbar, O.D., F.A.A.O.
ORS Founding Fellow
The evaluation for macular edema in diabetes has evolved since the advent of OCT. Clinically significant macular
edema is fading away, where as "center involved" macular thickness is becoming more important. The OCT does an
excellent job of helping identify those patients.
If you only look at the b-scans OCT you can miss macular edema that is not centered involved. In fact the b-scan can look normal.
One pearl is to also look at the thickness map. In some cases there can be thickening adjacent to the macula and this can be picked
up on the thickness map.
|

JOURNAL
ABSTRACTS
Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema.
This study funded by the National Institutes of Health evaluates the relative efficacy and safety of intravitreous aflibercept, bevacizumab, and ranibizumab in the treatment of diabetic macular edema. 89 clinical sites were randomly assigned 660 adults with diabetic macular edema involving the macular center to receive intravitreous aflibercept (224 participants), bevacizumab (218 participants), or ranibizumab (218 participants). An algorithm was used to determined the frequency of injections (as often as every 4 weeks). Visual acuity at 1 year was measured.
From baseline to 1 year, the mean visual-acuity letter score was measured (range, 0 to 100). When the initial visual-acuity was 20/40 or better (51% of participants), the mean improvement was 8.0 with aflibercept, 7.5 with bevacizumab, and 8.3 with ranibizumab. When the initial letter score was 20/50 or worse, the mean improvement was 18.9 with aflibercept, 11.8 with bevacizumab, and 14.2 with ranibizumab. There were no significant differences among the study groups in the rates of serious adverse events, hospitalization, death, or major cardiovascular events.
Therefore, intravitreous aflibercept, bevacizumab, or ranibizumab improved vision in eyes with center-involved diabetic macular edema, but the relative effect depended on baseline visual acuity. When the initial visual-acuity loss was mild (20/40 or better), there were no apparent differences, on average, among study groups. At worse levels of initial visual acuity (20/50 or worse), aflibercept was more effective at improving vision.
Wells J, Glassman A, Ayala A, et al. New England Journal of Medicine (The Diabetic Retinopathy Clinical Research Network), February 18, 2015.
Association of Smoking and CFH and ARMS2 Risk Variants with Younger Age at Onset of Neovascular Age-Related Macular Degeneration.
The importance of this study was to identify risk factors for an early age at onset of neovascular AMD. Since the age at which the first signs of age-related macular degeneration (AMD) manifest are variable, this study was performed to identify reasoning behind this. The results of the studies showed that past smokers and current smokers developed neovascular AMD on average 4.9 and 7.7 years earlier than non-smokers. The age of onset of wet AMD was also 5.2 years earlier for homozygous carriers of the A69s allele in the age-related maculopathy susceptibility 2 (ARMS2) gene. Patients that were homozygous carriers of the Y402H risk variant in the complement factor H (CFH) gene developed neovascular AMD 2.8 years earlier. And finally, patients carrying 4 risk allels in CFH and ARMS2 developed neovascular AMD 12.2 years earlier than patients with zero risk allels. The importance of this study showed that genetic and environmental risk factors influence the age at onset of neovascular AMD.
Lechanteur Y,MD; Camp P,Smailhodzic D, et al. JAMA Opthalmology 2015 February.
The Risk of Toxic Retinopathy in Patients on Long-term Hydroxychloroquine Therapy.
The objective of this study was to reassess the prevalence of and risk factors for hydroxychloroquine retinal toxicity and to determine dosage levels that facilitate safe use of the drug.
This was a retrospective case-control study in an integrated health organization of approximately 3.4 million members among 2361 patients who had used hydroxychloroquine continuously for at least 5 years according to pharmacy records and who were evaluated with visual field testing or spectral-domain optical coherence tomography.
Real body weight predicted risk better than ideal body weight and was used for all calculations. The overall prevalence of hydroxychloroquine retinopathy was 7.5% but varied with daily consumption and with duration of use. For daily consumption of 4.0 to 5.0 mg/kg, the prevalence of retinal toxicity remained less than 2% within the first 10 years of use but rose to almost 20% after 20 years of use. Other major risk factors include kidney disease and concurrent tamoxifen citrate therapy.
This study data suggest that hydroxychloroquine retinopathy is more common than previously recognized, especially at high dosages and long duration of use. While no completely safe dosage is identified from this study, daily consumption of 5.0 mg/kg of real body weight or less is associated with a low risk for up to 10 years.
Ronald B. Melles; Michael F. Marmor JAMA Ophthalmol. 2014;132(12):1453-1460.
Treatment Outcomes After 3 Years in Neovascular Age- Related Macular Degeneration Using a Treat-and-Extend Regimen.
This retrospective, interventional, consecutive case series determined the 3-year treatment outcomes after 1 to 3 years of ranibizumab or bevacizumab therapy using a treat-and-extend regimen in patients with neovascular age-related macular degeneration (AMD).
212 eyes from 196 patients diagnosed with treatment-naive neovascular AMD were treated with either ranibizumab or bevacizumab for a minimum of 1 year, using a treat-and-extend regimen. The main outcome measures were change from baseline best-corrected Snellen visual acuity (BCVA), proportion of eyes losing less than 3 BCVA lines, proportion of eyes gaining 3 or more BCVA lines, change from baseline central retinal thickness, and mean number of injections at 1, 2 and 3 years of follow-up.
At baseline, mean BCVA was 20/139, which improved to 20/79 after 1 year of treatment and was maintained at 20/69 and 20/64 at 2 and 3 years follow-up, respectively. At baseline, mean central retinal thickness was 351 mm and significantly decreased to 285 mm, 275 mm and 276 mm at 1, 2 and 3 years of follow-up, respectively. Patients received, on average, 7.6, 5.7 and 5.8 injections over years 1, 2 and 3 of treatment, respectively. At final follow-up, 94% of eyes had lost less than 3 lines BCVA, and 34.4% of eyes had gained 3 or more lines BCVA.
Therefore, the treat-and-extend regimen is effective in achieving and maintaining visual and anatomic improvements in patients with neovascular AMD for up to 3 years of treatment.
Rayess N, Houston SK 3rd, Gupta OP, et al. American Journal of Ophthalmology Volume 159 (1), Pages 3-8, Jan 2015.
Three-dimensional Vascular Imaging of Proliferative Diabetic Retinopathy by Doppler Optical Coherence Tomography.
This prospective, nonrandomized clinical trial evaluates the 3-dimensional architecture of neovascularization in proliferative diabetic retinopathy using Doppler optical coherence tomography (OCT).
Seventeen eyes of 14 patients with proliferative diabetic retinopathy were prospectively studied. Prototype Doppler OCT was used to evaluate the 3-dimensional vascular architecture at vitreoretinal adhesions. The results found that Proliferative membranes were detected in all eyes with proliferative diabetic retinopathy by standard OCT images. Doppler OCT images detected blood flow by neovascularization of the disc in 12 eyes and neovascularization elsewhere in 11 eyes.
The authors concluded that doppler OCT is useful for the detection and evaluation of the 3-dimensional vascular structure of neovascularization, and can assist in the noninvasive assessment of proliferative diabetic retinopathy.
Miura M, Hong Y, Yasuno Y, et al. American Journal of Ophthalmology Volume 159 (3), Pages 538-538, March 2015.
Geographic Atrophy In Patients Receiving Anti-Vascular Endothelial Growth Factor For Neovascular Age-Related Macular Degeneration.
The purpose of this study was to investigate factors associated with GA growth in neovascular ARMD patients receiving intravitreal anti-VEGF therapy on a treat and extend regimen.
This retrospective cohort study utilized two independent graders to identify GA using near-IR reflectance imaging and SD-OCT. FA was used to classify neovascular lesion subtypes as occult, classic, RAP, or mixed choroidal neovascularization as well as anatomical classification as Type 1 (sub-RPE), Type 2 (subretinal), Type 3 (intraretinal), or mixed neovascularization.
Out of 94 eyes included in the study, 52 eyes (55.3%) experienced apparent GA growth. Type 1 neovascularization showed significantly lower chance of developing GA than other neovascular lesion types. The study also showed that there is a stronger correlation between GA growth and subtype of neovascularization when the lesions are classified utilizing FA and SD-OCT.
Xu L, Mregen S, Jung J, et al. Retina. February 2015; 35(2):176-187.
Nondamaging Photothermal Therapy For The Retina: Initial Clinical Experience With Chronic Central Serous Retinopathy.
The purpose of this study was to evaluate the efficacy and safety of nondamaging PTT for the treatment of chronic CSR.
16 eyes with CSR for greater than 4 months duration were treated with PASCAL Streamline at 577 nm using 200 µm spot sizes. Endpoint Management Software was used to determine 100% pulse energy, and subsequently 30% pulse energy with spot spacing 0.25 beam diameter was applied over areas of serous RD as well as adjacent non-thickened retina. The study then measured changes in sub-retinal fluid, ETDRS BCVA and central macular thickness over a 6 month follow up period, along with pre and post-treatment FA and fundus autofluorescence.
An average of 532 spots were applied per treated eye. Post treatment, no laser marks were detectable by clinical observation, OCT, fundus autofluorescence or FA. An average of 12 ETDRS letters were gained at 2 months and sustained at 6 months. Central macular thickness decreased from 350 µm to 282 µm. SRF resolved completely in 37% of eyes after the first treatment. 44% of eyes required retreatment after 3 months due to recurrent or incomplete resolution of subretinal fluid. 19% of patients received a second treatment. 75% of eyes had complete resolution of SRF at 6 months, while the remaining 25% had some residual fluid.
In conclusion, PTT as utilized in this study was safe, improved VA and aided in the resolution of SRF in chronic CSR. The lack of tissue damage with PTT allows for retreatment without scarring unlike conventional photocoagulation. PTT should be studied in the treatment of other macular disorders as it may offer an alternative treatment to conventional laser coagulation as well as anti-VEGF injections.
Lavinsky D, Palanker D. Retina. February 2015; 35(2):213-222.
Increased Incidence Of Peptic Ulcer Disease In Central Serous Chorioretinopathy Patients: A Population-Based Retrospective Cohort Study.
The purpose of this study was to investigate possible risk factors for CSR, including peptic ulcer disease. Longitudinal data was analyzed in this population-based retrospective cohort study comprised of 835 patients with CSR and 4175 patients without CSR from January 2000 to December 2009.
The results of this study showed that peptic ulcer disease and higher monthly income were identifiable risk factors for CSR. Also, patients with CSR had significantly higher odds of developing peptic ulcer disease after they were diagnosed with CSR.
Chen S, Lian I, Chen Y, et al. Retina. February 2015; 35(2): 231-237.
Paravascular Inner Retinal Defect Associated With High Myopia or Epiretinal Membrane.
The goal of the study was to prospectively investigate the characteristics and pathogenesis of paravascular inner retinal defects (PIRDs). Paravascular retinal abnormalities are common in highly myopic eyes. However, affected areas may be underestimated, and the pathogenesis and effects on retinal function remain unclear.
Prospective and observational case series (between April 2013 and April 2014) was studied at a referral retinal practice among 28 patients (41 eyes) with PIRDs. The entire affected retinal area was examined in 4 quadrants in sequential thin sections using OCT. The effect of PIRDs on retinal function was examined using Goldmann perimetry.
On fundus photography, PIRDs appeared as spindle-shaped or caterpillar-shaped dark areas along the major retinal vessels disconnected from the optic disc. On OCT cross-sections of retinal vessels, PIRDs often appeared as cystoid or fissure-like spaces; however, longitudinal optical coherence tomography sections along retinal vessels revealed that most PIRDs were actually wide defects in the inner retina or located beneath the major retinal vessels, often deviating into the vitreous cavity. Of 41 eyes with PIRDs, 37 (90%) were myopic; 21 eyes (51%) had high myopia. The mean refractive error of the eyes with PIRDs was -7.94 diopters. The mean axial length of the eyes with PIRDs was 26.96 mm. Twenty-one eyes (51%) showed epiretinal membrane in the macular area. Of 41 eyes with PIRDs, 35 showed visual field defects corresponding to the PIRD locations. The most common visual field defects were relative Bjerrum scotoma and nasal steps corresponding to the PIRD predilection locations.
Paravascular inner retinal defects primarily occur in eyes with high myopia or epiretinal membrane. Paravascular inner retinal defects often cause retinal dysfunction corresponding to the location. A PIRD may partially overlap with retinal lesions previously reported as cleavage of the retinal nerve fiber layer, inner retinal cleavage, paravascular retinal cysts, or lamellar holes. However, the term PIRD more precisely describes the characteristic features of the lesion.
Yuki Muraoka, Akitaka Tsujikawa, Masayuki Hata, et al. JAMA Ophthalmol. Published online January 22, 2015.
One-Year Outcomes of Aflibercept in Recurrent or Persistent Neovascular Age-Related Macular Degeneration.
This retrospective, interventional, consecutive case series evaluates 6-month and 1-year outcomes of every-8-weeks (Q8W) aflibercept in patients with resistant neovascular age-related macular degeneration (AMD). Resistance was defined as multiple recurrences or persistent exudation with (Q4W) ranibizumab or bevacizumab that were switched to Q8W aflibercept.
Sixty-three eyes of 58 patients had a median of 13 previous anti-vascular endothelial growth factor (anti-VEGF) injections. 60.3% of eyes with resistant AMD while on Q4W ranibizumab or bevacizumab were completely dry after changing to Q8W aflibercept at the 6-month and 1-year follow-ups. There was no significant improvement in ETDRS visual acuity at 6 months and 1 year follow-up compared with baseline. Only a third of eyes needed Q4W aflibercept owing to persistence of fluid.
Therefore, Q8W dosing of aflibercept without the initial 3 monthly loading doses may be a good alternative in a select group of patients who may have developed ranibizumab or bevacizumab resistance.
Arcinue CA, Ma F, Barteselli G, et al. American Journal of Ophthalmology Volume 159 (3), Pages 426-436, Mar 2015.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
This 25 year old African American male has reactivated ocular toxoplasmosis. Ocular toxoplasmosis is caused by the parasite toxoplasmosis gondii,
and is the most frequent cause of infectious retinal disease and posterior uveitis.1
It is a disease with a variety of clinical presentations occurring in immunocompetent and immunocompromised patients, and whether it is acquired congenitally
or postnatally no clear distinction based on disease characteristics can be made.1
Classically, ocular toxoplasmosis presents as a well-defined area of retinal necrosis accompanied by vitritis.1,2
The vitritis is commonly seen overlying the active lesion and can be severe enough to prevent good visualization of the lesion (i.e. "headlights in the fog"). There is often diffuse
inflammation in the neighboring retinal and choroidal tissue, thus it is described as a "necrotizing retinochoroiditis."1
In patients with a competent immune system active lesions will "heal" within 2-4 months resulting in a hyperpigmented, atrophic scar. Anterior uveitis, retinal vasculitis and optic nerve
swelling may also be present. Often acute ocular toxoplasmosis lesions are associated with adjacent old scars, described as satellite lesions, indicating a recurrent attack.1 Depending
on the location of the lesion and amount of inflammation present, patients may be very symptomatic with floaters, decreased vision, or photophobia or as in this case, have no symptoms at all.
Ocular complications can include choroidal neovascular membranes, vascular occlusions, retinal breaks or detachments, macular edema, optic atrophy, synechiae, cataracts and
glaucoma.1
Toxplasmosis gondii exists in three forms: (1) oocyst (eggs of the parasite) (2) tachyzoite and (3) bradyzoite (tissue cyst). During primary infection which occurs in felines, millions
of oocysts are shed in their feces. Humans can become infected by intrauterine infection, inhaling oocysts in cat litter or ingesting contaminated water, soil or food. When oocysts are
ingested, tachyzoites form, rapidly multiplying and invading host cells. The tachyzoites cause an intense inflammatory response with tissue destruction and are responsible for the clinical
findings of the disease. Under pressure from the immune system, tachyzoites are transformed into bradyzoites that form
cysts.1 Tissue cysts are found in the retina, brain, skeletal and heart muscles. They are very
resistant and can remain dormant in tissue without causing damage for many years. For unknown reasons, they can rupture, causing reactivation of disease. Humans may also
be infected by ingesting bradyzoite tissue cysts found in raw and undercooked meat.1,3 Transmission
can also occur in organ transplantation and blood transfusion.3 However, not everyone who is infected
with Toxoplasmosis gondii develops eye disease. Host genetic factors may determine whether an infection results in ocular disease.4
Differential diagnosis includes congenital diseases of the ToRCHS acronym namely rubella, cytomegalovirus, herpes viruses and syphilis. Additionally tuberculosis posterior
uveitis, neuroretinitis, acute retinal necrosis, diffuse unilateral subacute neuroretinitis (DUSN), toxocariasis, Behcet disease, white dot syndromes, and primary vitreoretinal
lymphoma are included.5
The diagnosis of toxoplasmosis is a clinical one based on classic fundus findings. However, atypical presentations such as neuroretinitis, scleritis, a large active lesion without
a scar, or punctate outer retinal toxoplasmosis (PORT) which involves extensive, deep retinal layers without much vitritis or adjacent scars, can occur.1
Diagnostic testing can be helpful to either confirm clinical impressions or differentiate atypical presentations which may look like other causes of autoimmune or infectious
posterior uveitis. Enzyme-linked fluorescence assay (ELISA) toxoplasmosis antibody test of the blood, aqueous humor or vitreous can discriminate between IgG, IgM and IgA antibodies.
If IgM and/or IgA antibodies are present it indicates an acute infection versus IgG antibodies are detected if the infection was acquired more than 4 months prior.1 Definite confirmation
is obtained in aqueous humor or vitreous fluid samples by polymerase chain reaction (PCR) to detect toxoplasmosis gondii DNA.1
There is no universally accepted treatment approach for ocular toxoplasmosis.2,6,7 Current therapies do not eliminate
tissue cysts and cannot prevent chronic infection. The decision of what medications or even if ocular toxoplasmosis needs treatment is controversial.7
It is generally agreed that indications for treatment include: lesions within the vascular arcades (because they threaten the macula), lesions close to the optic nerve
(because substantial visual field loss can occur), larger lesions > 2 disc diameters, and lesions in immunosuppressed individuals (because untreated often develop recalcitrant,
progressive disease). Regarding peripheral, non-sight threatening lesions, as in the photo above, treatment decisions are more variable. Some eye care providers will choose not to
treat these lesions as they are often self-limited, while others will choose to treat all lesions regardless of location.1,7
The standard therapy for toxoplasmosis has been "triple therapy" with sulfadiazine, pyrimethamine, and prednisone for more than 60 years. Trimethoprim-sulfamethoxazole (TMP-SMX), systemic
or intravitreal clindamycin with dexamethasone, and azithromycin with pyrimethamine have all been compared against standard therapy and have shown no difference in outcomes, including rate
of recurrence, time to resolution, or final visual acuity.7 Some of these treatments have significant systemic side
effects such as bone marrow suppression and pseudomembranous colitis and it recommended to monitor these patients with an internist.7
Prompt referral to a retinal specialist is advised in active, vision threatening lesions or atypical presentations. Anterior segment inflammation may be managed with topical
cycloplegics and/or steroids.1
1. Maenz M, Schluter D, Liesenfeld O et al. Ocular toxoplasmosis past, present and new aspects of an old disease. Progress in Retinal and Eye Research 2014; 39:77-106.
2. Harrell M, Carvounis PE. Current treatment of toxoplasma retinochoroiditis: an evidence-based review. Journal of Ophthalmology 2014;
3. Pleyer U, Schluter D, Manz M. Ocular toxoplasmosis: recent aspects of pathophysiology and clinical implications. Ophthalmic Res 2014;52:116-123.
4. Kijlstra A and Petersen E. Epidemiology, pathophysiology, and the future of ocular toxoplasmosis. Ocular Immunology & Inflammation 2014;22(2):138-147.
5. Vasconcelos-Santos DV, Dodds EM, Orefice F. Review for disease of the year: differential diagnosis of ocular toxoplasmosis. Ocular Immunology & Inflammation 2011;19(3):171-179.
6. Felix JF,Lira RPC, Zacchia RS et al. Trimetoprim-sulfamethoxazole versus placebo to reduce the risk of recurrences of toxoplasma gondii retinochoroiditis: randomized controlled
clinical trial. Am J Ophthalmol 2014;157(4):762-766.
7. Schallhorn JM, Gonzales J. Ocular toxoplasmosis: the treatment dilemma. Journal of AAPOS 2013;17:454-455.
|

IN THE
NEWS
 |
FDA, EMA Grant Nightstar Orphan Drug Designation For Gene Therapy To Treat Choroideremia
Nightstar has received orphan drug designation from the FDA and the European Medicines Agency for a gene
therapy to treat choroideremia, according to a press release.
The designation will provide regulatory support in development activities such as protocol assistance, reduced fees,
tax incentives and market exclusivity for the product upon approval, the release said.
"We are committed to finding effective treatments for retinal dystrophies, and the support and assistance of the regulatory
bodies in the U.S. and Europe will be invaluable in our further development work," David Fellows, CEO of Nightstar,
said in the release.
In a study published in The Lancet, six patients showed subjective improvement in dim light and two of the six patients
were able to read more than two lines on an eye chart 6 months after the gene therapy treatment, the release said.
|
 |
Clearside Launches Phase 2 Clinical Trial For Triamcinolone Acetonide
Clearside Biomedical has begun enrollment for a phase 2 clinical trial to evaluate triamcinolone acetonide in the treatment
of macular edema associated with retinal vein occlusion, according to a press release.
Clearside's proprietary formulation of triamcinolone acetonide, CLS-TA, is administered via suprachoroidal injection with
the company's micro-injector.
The multicenter, randomized, controlled trial is designed to gauge the safety and efficacy of a single suprachoroidal injection
of CLS-TA combined with a single intravitreal injection of Eylea (aflibercept, Regeneron) vs. an intravitreal injection of
Eylea alone.
The study will include about 40 patients enrolled at 10 sites in the U.S. Patients will receive one intravitreal injection
of aflibercept and be randomized 1:1 to receive a suprachoroidal injection of CLS-TA or sham treatment during one visit.
Subsequently, patients will visit the clinic once a month for 3 months.
Patients in either treatment arm with persistent macular edema or reduced visual acuity will be evaluated at 1, 2 or 3 months
after initial treatment to receive additional intravitreal injections of aflibercept.
The study's main goal is to determine if a single suprachoroidal injection of CLS-TA combined with an intravitreal injection
of aflibercept, as opposed to a single intravitreal injection of aflibercept alone, will reduce the need for
additional intravitreal aflibercept injections over 3 months.
|
 |
Poor Anti-VEGF Response May Be Linked To Obstructive Sleep Apnea In Patients With AMD, DME
Patients with exudative age-related macular degeneration or diabetic macular edema who responded poorly to anti-VEGF therapy
may have an elevated risk of obstructive sleep apnea, according to a study in Retina.
The case-control study included 103 patients diagnosed with AMD and 77 patients diagnosed with DME. A control group comprised 100 volunteers.
Patients with AMD or DME underwent as-needed injections of Avastin (bevacizumab, Genentech). Criteria for re-injection were loss
of visual acuity of more than five letters, evidence of macular fluid, increase in central macular thickness of at least
100 µm, new macular hemorrhage, classic choroidal neovascularization or evidence of persistent fluid at 1 month. All
patients were examined every 6 weeks for at least 1 year.
Fifty-six AMD patients (54.37%) had non-exudative AMD, and 47 AMD patients (45.63%) had exudative AMD. Among the
47 patients with exudative AMD, 14 (29.79%) responded poorly to anti-VEGF therapy and had a significantly higher risk
of obstructive sleep apnea (P < .05).
In the DME group, among 30 patients with cystoid macular edema, a higher number of injections was significantly associated with an increased risk of obstructive sleep apnea (P < .05).
|
 |
Good Results Seen With Air Injection, Tissue Plasminogen Activator In Eyes With Submacular Hemorrhages Due To AMD
Subretinal air injection with tissue plasminogen activator and postoperative positioning may improve the outcome of
massive submacular hemorrhages secondary to age-related macular degeneration, according to a study in Ophthalmology.
The prospective, interventional case series included 13 eyes of 13 consecutive patients with massive submacular hemorrhages
with a 3-month follow-up after the procedure.
After vitrectomy, pneumatic displacement involved a submacular injection of 0.4 mL of tissue plasminogen activator and 0.4 mL
of filtered air using a microneedle. The patient stayed in the prone position overnight so that the air pressure would displace
the hemorrhage.
All eyes achieved total subfoveal blood displacement.
One month postoperatively, visual acuity improved by more than two lines in 11 eyes, was unchanged in one eye and worsened in
one eye. Three months postoperatively, visual acuity improved by more than five lines in 11 eyes and decreased by more than
two lines in one eye.
Mean visual acuity in all eyes improved from 20/300 at baseline to 20/130 at 1 month after surgery and 20/100 at 3 months.
Mean OCT central lesion thickness decreased from 796 µm at baseline to 361 µm at 3 months postoperatively (P = .0003).
Surgical complications included intraoperative macular hole formation in one eye and vitreous hemorrhages in one eye.
|
 |
Ready-Diluted Formulation Of Jetrea Receives Positive Opinion In European Union
The Committee for Medicinal Products for Human Use of the European Medicines Agency has granted a positive opinion for
a ready-diluted formulation of Jetrea, according to a press release from ThromboGenics.
Jetrea (ocriplasmin) is approved in the European Union for the treatment of vitreomacular traction (VMT). The new formulation
is designed to eliminate the preparatory dilution steps before injection.
"With this new formulation, it will be easier and more convenient for retina physicians to use this novel pharmacological
option for the treatment of patients with VMT," Patrik De Haes, MD, CEO of ThromboGenics, said in the release.
ThromboGenics anticipates final approval from the European Commission in 2 to 3 months. Alcon, which commercializes Jetrea
outside of the U.S., will be responsible for planning the launch of the new formulation, the release said.
|
 |
Santen Seeks European Marketing Authorization For Intravitreal Sirolimus
The European Medicines Agency has accepted a marketing authorization application from Santen Pharmaceutical for the use
of intravitreal sirolimus 440 µg for the treatment of noninfectious uveitis of the posterior segment, the company announced in a press release.
The application is backed by key outcomes data from the SAKURA study. These data include proportion of patients achieving
a vitreous haze score of zero at 5 months, proportion of patients attaining a vitreous haze score of zero or 0.5+ at 5 months,
proportion of subjects attaining an improvement in vitreous haze score of two units and proportion of patients successfully
tapering off systemic corticosteroids at 5 months, the release said.
|
 |
Vitrectomy For Lamellar Macular Hole May Not Require Air Tamponade
Air tamponade may not be necessary to improve vision in eyes with lamellar macular hole that undergo vitrectomy, according to
a study in Ophthalmic Surgery, Lasers, and Imaging.
The retrospective study included 41 eyes of 39 patients who underwent 25-gauge vitrectomy with air tamponade (23 eyes) or
without air tamponade (18 eyes).
Average baseline logMAR best corrected visual acuity was 0.26 in the tamponade group and 0.35 in the no tamponade group.
OCT was used to confirm the presence of a lamellar macular hole before and after surgery. Standard three-port vitrectomy
was performed in all cases.
At 6 months, postoperative BCVA was 0.12 in the tamponade group and 0.14 in the no tamponade group. BCVA improved significantly
in both groups (P = .023 in the tamponade group and P < .001 in the no tamponade group).
BCVA was similar in both groups before and after surgery.
Relationships between BCVA and hole diameter and foveal thickness of the lamellar macular hole were insignificant.
No cases of postoperative full-thickness macular hole were identified in either group.
|
 |
Choroidal Thickness Reduced In Most Regions Of Eyes With Full-Thickness Macular Holes
Choroidal thickness was reduced in eyes with full-thickness macular holes and contralateral eyes with vitreomacular
adhesion, according to a study Ophthalmic Surgery, Lasers, and Imaging.
The retrospective study included 19 patients with a unilateral full-thickness macular hole and contralateral
vitreomacular adhesion.
An age-matched control group comprised 19 healthy patients.
Three groups were analyzed: full-thickness macular hole, contralateral vitreomacular adhesion and controls.
Optical coherence tomography was used to measure choroidal thickness subfoveally and 1 mm and 2 mm away from the fovea
in the nasal and temporal sectors.
Median subfoveal choroidal thickness was 197 µm in the full-thickness macular hole group, 215 µm in the contralateral
vitreomacular adhesion group and 262 µm in the control group.
The differences between the macular hole and vitreomacular adhesion groups and macular hole and control groups were
statistically significant (P = .0013).
Differences in choroidal thickness were significant 1 mm nasal from the fovea between the macular hole and control groups
and vitreomacular adhesion and control groups; 2 mm nasal from the fovea between the macular hole and control groups
and vitreomacular adhesion and control groups; and 1 mm temporal from the fovea between the macular hole and control groups.
There were no significant differences 2 mm temporal from the fovea.
The study authors said the findings suggest "an association between choroidal thinning and macular hole."
|

MEET
THE FELLOWS
In each issue, a Fellow of the Optometric
Retinal Society is highlighted. In this issue, Dr. Stephanie Klemencic, a new ORS fellow, is highlighted.
Dr. Stephanie Klemencic is a graduate of the Indiana University School of Optometry and completed a residency in Ocular Disease
from the same institution. She worked in a private OD/MD practice in Ohio before joining the faculty at the Illinois College
of Optometry (ICO) in 2007. She currently is an Associate professor and coordinator of ICO's primary eye care and ocular
disease residency program where she provides mentorship and education to five residents each year. Her primary focus is in
clinical patient care where she educates students and residents in primary care, advanced ocular disease, and ocular
emergencies clinics. Her clinical interests are in all aspects of ocular disease management with a focus in retina and glaucoma.
Dr. Klemencic is a fellow of the American Academy of Optometry, Optometric Retina Society and an American Board of
Optometry diplomate. In addition to her clinical duties, she lectures in Retina, Neurophthalmic Disorders, and Ocular
Emergencies courses at ICO, has authored multiple publications on ocular disease topics, and presents continuing education
courses at local, state and national levels.
Dr. Klemencic lives in Chicago with her husband, daughter, and a neurotic Doberman pincher named Isa. In her spare time she
enjoys spending time with her family, trying to get better at golf, and traveling the world on wine tasting tours.
WHY
BECOME AN ORS MEMBER?
By Rex Ballinger, O.D., F.A.A.O.
Chair, Membership Committee
Membership in the Society can provide several benefits. You may
receive discounts at annual meetings. You’ll receive regular
newsletters on new and exciting updates on retinal disease diagnosis
and management as well as other newsy items of interest. And you’ll
be associated with a body of knowledge and resources which can
help you in many other ways. So consider membership in the Society.
It will be worth your while in your quest for better understanding
of the retina.
If your interests extend beyond the general, if you want to become
part of the dynamic team involved in the Society to share your
interest and enthusiasm with your colleagues, consider becoming
a Fellow member. Details and applications can be found at
www.optometricretinasociety.org |

SPONSOR NEWS
Scientifically Proven AMD & Visual Performance Product
MacuHealth with LMZ3
is a patented natural eye health supplement consisting of the three primary
protective pigments found in the macula. Taking this one small, easy-to-swallow soft gel
once a day has been clinically proven to be the best product for restoring macular
pigments to normal levels for enhanced vision and help in
the prevention of age-related macular degeneration and Visual Performance Improvement in Healthy eyes.
Download VEE Speaker Series
www.macuhealth.com

Editor
in Chief
Steven Ferrucci, OD, FAAO
Co-Editor
Mark T. Dunbar, OD, FAAO |
Journal
Reviewers
Tahrim Rahman, OD
Kristie Draskovic, OD
Savannah Brunt, OD
Nicholas Zagorianos, OD
Art/Production Director
Joe Morris
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click
here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click
here if you do not want to receive future emails from Review of Optometry. |
|










