| |
Volume 11, Number 2 |
May
2015 |
|
|
Inside
This Issue
|
|
|
|
|
|
This e-newsletter is provided free to doctors through industry support from |
 |
| |
FROM
THE DESK OF THE EDITOR
Last week, a patient called my office asking if he could get a pair
of glasses he heard about that could "cure his macular degeneration". I called the patient to discuss with him that no
such glasses exist, but we would be happy to examine him again (it had been about 2 years) and see if there was
anything further we could offer for his rather advanced AMD. He declined, as he had an outside retinal specialist, and
was still happy with the low vision devices he received 2 years ago. He just wanted those damn glasses!! Turns out,
he heard about them on Dr. Oz! Now, make no mistake about it, I am not a Dr. Oz fan. I have long felt he offers advice that
is misleading at worst, or at best has very little medical basis. I have heard him make several statements about eye care
that is simply erroneous. Apparently, I am not alone. There has been recent backlash against his show, and some have
suggested he lose his license or at least give up his University appointment due to some of his misleading claims.
LIVE
POLL
|
How do you diagnose clinically significant macular edema (CSME) in a diabetic patient?
|
|
A friend told me about an article that compares the medical advice
given by Dr. Oz with that of another TV doctor, Doc McStuffins. For those of you who may not have children, the Disney
Junior series chronicles a girl who decides she wants to become a doctor like her mother. She pretends to be a doctor
by fixing toys and dolls. When she puts on her stethoscope, toys, dolls, and stuffed animals come to life and she
can communicate with them. With help from her stuffed animal friends — Stuffy the Dragon, Hallie the Hippo,
Lambie the Lamb, and Chilly the Snowman — Doc helps toys "feel better" by giving them check-ups and diagnosing
their illnesses.
The article references a study in which researchers watched 5 episodes of each show, Dr. Oz and Doc McStuffins, and evaluated
the validity of each medical claim made on the shows. Each individual claim was rated on a scale of 5 ("strongly supported by
the literature") to -5 ("directly contra-indicated by the literature") with 0 representing "insufficient data to support or refute
the claim". Then the results were tallied, and an average score calculated. With a score of 0.57 to -3.73 DocMcStuffins was the
more science-based show of the two.
Obviously, I relate this study in jest (or do I?) but the point is that patients can get bad information from anywhere: the
internet, friends, television, magazines or even other Doctors. It is our job to explain the limitations due to our
patients conditions, and often explain that not everything they hear is correct, or such a treatment is not indicated in their
case. The realty can unfortunately be harsh at times, but we owe it to our patients to be honest and direct, so they understand
what they can realistically expect from their vision or condition moving forward. Harsh reality is always better than false hope.
Steven Ferrucci, O.D., F.A.A.O.
Editor in Chief
|
ORS/REVIEW OF OPTOMETRY MEETING
We are excited about bring the ORS/Review of Optometry Retina Update back to the west coast this year!
A distinguished slate of speakers from both optometry and ophthalmology will present clinically relevant information on
vitreoretinal disease prevention, diagnosis, technology, and treatment/management. Don't miss optometry's premier posterior
segment educational event as we focus on providing education and insight into best retina practices in 2015.
Click
here for more information and to register.
Joe Pizzimenti O.D., F.A.A.O.
Conference Co-chair
ORS Fellow
Past-President
|
PRESIDENT'S MESSAGE
When it comes to diagnosing and managing retinal disease, I am truly thankful to be practicing in an era of
unparalleled advancement in retinal imaging. Over the last several years, SD OCT, Fundus Autofluorescence and
Multispectral Imaging have become critical tools in our arsenal. Continued advancements in digital photography have
resulted in amazing photographic capabilities, including improvements in time tested techniques such as angiography.
MARCH 2015
POLL RESULTS
 |
In Europe, OCT instruments are being utilized to perform a version of "dyeless angiography", and the
first commercial Swept Source OCT is being marketed. It is hard to conceive of providing high quality patient
care without the bells and whistles available to us today. But you know what else I am thankful for? I am
thankful that I began practicing BEFORE most of these instruments were available.
I have been out of Optometry School for 22 years, having graduated in 1993. I was fortunate enough to undertake
residency training at an Omni referral center in Memphis, then stay there as the center director for six years.
Patients from all over the Mid-South were referred to us for consultation, often due to retinal pathology.
In order to evaluate these patients, we had three, maybe four, things at our disposal. We could examine them, we could
take a retinal photograph, and we could perform Fluorescein Angiography. In some circumstances, B-scan ultrasonography
was useful. No OCT, no Fundus Autofluorescence, no spectral imaging.
We looked at a lot of retinas, and performed a lot of angiograms. But because we did not have the toys that we have today,
I had to be good at seeing CME, diabetic CSME, lamellar macular holes, epiretinal membranes, etc. Our observational
skills were critical.
We could photograph what we saw for future reference, but as far as learning anything else about what might be going
on, relatively invasive Fluorescein Angiography and maybe a B-scan in the right situations were all that we had. We
simply had to see the problem.
I am genuinely thankful to have practiced at a time and in a setting where that was the case, and I worry sometimes
about the students that I work with every day. They have everything at their fingertips, but will they be able to see?
Brad Sutton, O.D., F.A.A.O.
ORS President

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.

|
CLINICAL
PEARLS
OCT Interpretation
By Mohammed Rafieetary, O.D., F.A.A.O.
ORS Fellow
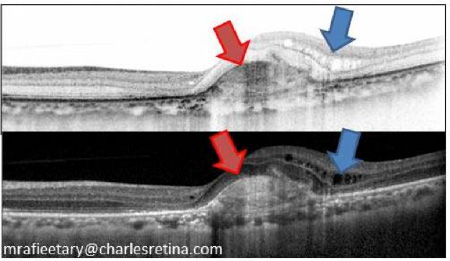
In interpreting OCT scans, most clinicians have the tendency to make a specific diagnosis based on the alteration of
the retinal tissue. Instead when unsure of the specific disease entity the finding can be described based on
the morphologic findings. For example in the image below the area marked by the red arrow (In both black on white or
white on black scan lines) is a choroidal neovascular membrane, however it can be described as an outer segment area
of "Hyperreflectance" or "Hyperreflectivity" indicating tissue alteration denser than what is expected. Conversely,
the area noted with blue arrow demonstrating intraretinal fluid can be describes as areas of "Hyporeflectance"
or "Hyporeflectivity" indicating absence of normal tissue.
Drusen and Choroidal Lesions
By Aaron Gold, O.D., F.A.A.O.
ORS Fellow
Drusen overlying a choroidal pigmented lesion does NOT necessarily rule out choroidal melanoma. On several occasions, I've heard
colleagues say something along the lines of, "I spotted a suspicious looking nevus, but there was also drusen, so it's benign." Although I agree
that drusen over a suspicious choroidal lesion is a good sign, as it indicates tumor chronicity, long dormant lesions may still undergo
malignant transformation on rare occasions. Because of this, be extra suspicious of choroidal lesions that present with drusen that appears to be
tightly concentrated over only one small area of the tumor.
|

JOURNAL
ABSTRACTS
Results And Prognostic Factors For Visual Improvement After Pars Plana Vitrectomy For Idiopathic Epiretinal Membrane.
The purpose of this article was to report postoperative visual improvement and prognostic factors of PPV for ERM.
504 eyes of 495 patients were studied from 2003 to 2012 with OCT, BCVA and demographic data. Outcome measures included
the proportion of eyes with improvement of BCVA of at least two Snellen lines at 3,6 and 12 months after PPV and
prognostic factors for improvement.
At three months post-op, 41.7% of eyes gained at least 2 lines, 34.7% at 6 months and 48.1% at 12 months.
Better prognosis at 3 months was reported with poorer pre-op BCVA and pseudophakia.
The investigators suggested that the lower rate of BCVA improvement for phakic patients with good pre-operative BCVA
may influence final visual improvement after PPV/ERM peel, and thus may influence the recommendation for surgery.
Song, SJ, Kuriyan A, Smiddy W. Retina. May 2015; 35(5):866-872.
Evolution Of Controlling Diabetic Retinopathy: Changing Trends In The Management Of Diabetic Macular Edema At A Single Institution Over The Past Decade.
The purpose of this paper was to report the evolution of treatment in patients with DME in a clinical setting.
This study was a retrospective observational case series of 1,862 patients treated for DME over the last
decade. Treatment modalities, VA, and degree of DME on OCT were recorded.
This study found that there was a linear decrease in focal laser use and exponential increase in intravitreal injections
for treatment of DME. Clinic visit frequency increased from 3 ± 2 visits per year to 9 ± 2 visits per year.
Visual and anatomical improvements were significant over the decade studied, with mean improvement in VA from less than
1 Snellen line early in the study to 2 Snellen lines with current treatment modalities. Decrease in retinal thickness
showed similar improvement with a change from 58 ± 59 µm to 162 ± 91 µm.
This studies' management of DME over the last decade evolved by replacement of focal laser therapy with
intravitreal injections. The investigators suggested this change produced significant improvement in VA and anatomy
in patients with DME, but has increased the frequency of office visits.
Jusufbegovic D, Mugavin M, Schaal S. Retina. May 2015; 35 (5):929-934.
Sustained Elevation Of Intraocular Pressure After Intravitreal Anti-VEGF Agents: What Is The Evidence?
The purpose of this article was to review and analyze past literature regarding elevation of IOP due to
intravitreal anti-VEGF injections in patients with wet ARMD.
Studies show that patients may experience sustained and delayed elevation of IOP after intravitreal anti-VEGF treatment.
The incidence of sustained IOP elevation varied from 3.45% to 11.6%, with few patients required surgery to
control IOP. Proposed risk factors for elevated IOP were extensive, including history of glaucoma, phakia, steroid use
and extended treatment duration. Multiple theories explaining the pathogenesis of elevated IOP have been proposed,
including microparticle obstruction of the trabecular meshwork, intraocular inflammation and transient elevation of IOP.
The investigators in this study suggested that sustained and delayed IOP elevation after intravitreal anti-VEGF is likely
a multifactorial process. They suggested further studies to prospectively investigate sustained elevation of IOP to
better understand the long-term effects associated with intravitreal anti-VEGF therapy.
Dedania V, Bakri S. Retina. May 2015; 35(5):841-858.
Correlation Between Fundus Autofluorescence and Central Visual Function in Chronic Central Serous Chorioretinopathy.
This prospective, cross-sectional study investigates possible correlations between the morphologic macular
changes revealed by fundus autofluorescence (FAF) and the functional parameters such as visual acuity and
retinal sensitivity in patients with chronic central serous chorioretinopathy (CSC).
Forty-six eyes with chronic CSC were studied with FAF and microperimetry (MP) with central 10-degree visual field.
Retinal sensitivity value maps were exactly superimposed over FAF images. Mean best-corrected visual acuity (BCVA) was
20/32 (range 20/20-20/200). BCVA was significantly correlated with FAF findings. A positive concordance between FAF and
MP evaluation was also found. The hypo-autofluorescent areas showed decreased retinal sensitivity, while adjacent areas
of increased FAF could be associated to both normal and decreased retinal sensitivity. Absolute scotoma, defined as
0 dB retinal sensitivity, corresponded with absence of autofluorescence.
Therefore, altered FAF in chronic CSC patients has a functional correlation quantified by microperimetry. This
study confirms the impact of FAF changes on retinal sensitivity and their value to reflect the functional impairment
in chronic CSC.
Eandi, Chiara M. et al. American Journal of Ophthalmology, Volume 159, Issue 4, 652-658.e1.
Reticular Pseudodrusen Associated With a Diseased Bruch Membrane in Pseudoxanthoma Elasticum.
Reticular pseudodrusen (RPD) pathophysiology remains incompletely understood. It is frequently associated with
Age Related Macular Degeneration but is considered an independent risk factor. This study was conducted to
identify the associations of RPD with other diseases that have defined pathophysiologic mechanisms
like Pseudoxanthoma Elasticum (PXE). The objective of this study was to describe the phenotype, prevalence,
and topographic distribution of RPD in patients with pseudoxanthoma elasticum (PXE) and their association with
a diseased Bruch membrane. The participants of this study were 42 patients with confirmed PXE diagnosis
through genetic testing and/or skin biopsy.
RPD were defined as irregular networks of round to oval lesions that appear hyporeflective on near-infrared
reflectance, hypoautofluorescent on fundus autofluorescence, and as subretinal deposits on spectral-domain optical
coherence tomographic images. The presence of RPD was judged based on characteristic findings in at least 2 of the
3 imaging modalities.
42 patients with PXE, RPD were detected in 22 patients. The study showed the prevalence of RPD was highest in the
fifth decade at 67%. The RPD were most frequently located within the superior quadrant and least frequently
located within the central macula. The RPD were always located central to areas with peau d'orange and within an area
of hypofluorescence on late-phase indocyanine green angiographic images.
The study data suggest that RPD have a high prevalence in eyes of patients with PXE. Although RPD in patients with PXE
occur at a younger age, their distribution and phenotype appear to be similar to RPD associated with age-related
macular degeneration. The association with diseased Bruch membrane in PXE suggests a pathogenetic role of Bruch
membrane alterations for the development of RPD.
Martin Gliem, Doris Hendig, Robert P. Finger et al. JAMA Ophthalmol.
2015;133(5):581-588. doi:10.1001/jamaophthalmol.2015.117.
An Insight Into the Pathogenesis of Optic Disc Pit–Associated Maculopathy With Enhanced Depth Imaging.
The goal of this study was to analyze the morphologic changes seen in the optic disc pit and evaluate the source
of subretinal fluid.
Four patients participated in this study and received complete ophthalmic evaluations, with fundus color photography
and enhanced depth imaging spectral-domain optical coherence tomography scanning of the optic disc. The optical
coherence tomographic section was mapped with infrared image and color photography, and the characteristics of the
retina and optic nerve head were analyzed. All the cases had outer layer retinal schisis; 2 of them had associated
serous macular detachment while inner retinal schisis was present in 3 cases. A hyporeflective tract was observed in
our study connecting the retinal schisis cavity and gap in the lamina cribrosa corresponding to the optic pit.
The study demonstrated the connectivity between retinal schisis and the gap in the lamina cribrosa present in the optic
disc pit, supporting the hypothesis of cerebrospinal fluid as the source of subretinal fluid.
Jaitra P. Gowdar, Bindu Rajesh, Anantharaman Giridhar et al. JAMA Ophthalmol.
2015;133(4):466-469. doi:10.1001/jamaophthalmol.2014.6093.
Choroidal Thickness Changes After Photodynamic Therapy and Recurrence of Chronic Central Serous Chorioretinopathy.
This study was conducted to investigate long-term changes in subfoveal choroidal thickness (SCT) after photodynamic
therapy (PDT) and their relationship with chronic central serous chorioretinopathy (CSC) recurrence.
The study investigated 57 eyes with chronic CSC for two years that were treated with half-dose PDT. There were 2 groups
in this study: those with incomplete CSC resolution or subretinal fluid (SRF) recurrence (SRF+) and those with complete
SRF absorption without disease recurrence (SRF-). The SCT was measured using spectral-domain optical coherence
tomography and relative SCT ratios (follow-up SCT: baseline SCT ratio) were compared between the 2 groups.
Four of 57 eyes had persistent SRF after PDT and 12 of 53 eyes had initial SRF resolution with SRF recurrence.
The SRF+ group had a slower reduction in SCT during the first month and a higher relative SCT ratio than the SRF-
group throughout follow-up. The relative SCT ratio at 1 month was highly predictive of CSC recurrence.
The study concluded that those with incomplete SRF absorption or SRF recurrence had a slower SCT decline at 1 month and
a higher SCT ratio throughout follow-up compared to those without CSC recurrence. The SCT changes may reflect PDT
efficacy and help predict long-term recurrence and early treatment outcomes.
Yong-Kyu Kim, Na-Kyung Ryoo, Se Joon Yoo et al. AJO: Published online: April 14, 2015.
Prevalence of Intermediate-Stage Age-Related Macular Degeneration in Patients With Acquired Immunodeficiency Syndrome.
This cross-sectional study evaluate the prevalence of intermediate-stage age-related macular degeneration (AMD)
in patients with acquired immunodeficiency syndrome (AIDS). Masked graders evaluated retinal photographs using
the Age-Related Eye Disease Study grading system. The study involved 1825 patients with AIDS and no
ocular opportunistic infections. Overall, 9.9% had intermediate-stage AMD. Risk factors included age;
the prevalence of AMD ranged from 4.0% for participants 30-39 years old to 24.3% for participants
≥60 years old. Other risk factors included the HIV risk groups of injection drug use or heterosexual
contact. Compared with the HIV-uninfected population in the Beaver Dam Offspring Study, there was an
approximate 4-fold increased age-adjusted prevalence of intermediate-stage AMD.
In conclusion, patients with AIDS have an increased age-adjusted prevalence of intermediate-stage AMD compared with
that found in a non-HIV-infected cohort evaluated with similar methods.
Jabs, Douglas A. et al. American Journal of Ophthalmology, Volume 159, Issue 6, 1115-1122.e1.
One-Year Result of Aflibercept Treatment on Age-Related Macular Degeneration and Predictive Factors for Visual Outcome.
This prospective nonrandomized interventional case series studies the efficacy of periodic injection of aflibercept
in different subtypes of age-related macular degeneration (AMD) while investigating predictive factors for
visual outcome in clinical settings.
The study contained 98 patients: 46 had typical AMD, 42 had polypoidal choroidal vasculopathy (PCV), and 10 had
retinal angiomatous proliferation (RAP). Patients received injections of aflibercept once a month for 3 months followed
by once every 2 months for 8 months.
The logarithm of the minimal angle of resolution (logMAR) at 12 months and improvement of vision from baseline were
compared the different AMD subtypes. Mean logMAR improved from 0.36 to 0.21 in 12 months. There was no difference in
visual improvement between typical AMD and PCV, however final logMAR was better in PCV (0.32 vs 0.08,
P = .016). Follow-up angiography showed regression of polyps in PCV in 69.2% of cases. Multiple
regression analysis showed that the presence of external limiting membrane (ELM), smaller greatest linear dimension,
and the presence of polypoidal lesion were associated with better visual outcome.
The study concluded that periodic injection of aflibercept is effective for PCV as well as for typical AMD. The statuses
of ELM, greatest linear dimension, and polypoidal lesion are predictive for visual outcome.
Oishi, Akio et al. American Journal of Ophthalmology, Volume 159, Issue 5, 853-860.e1.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
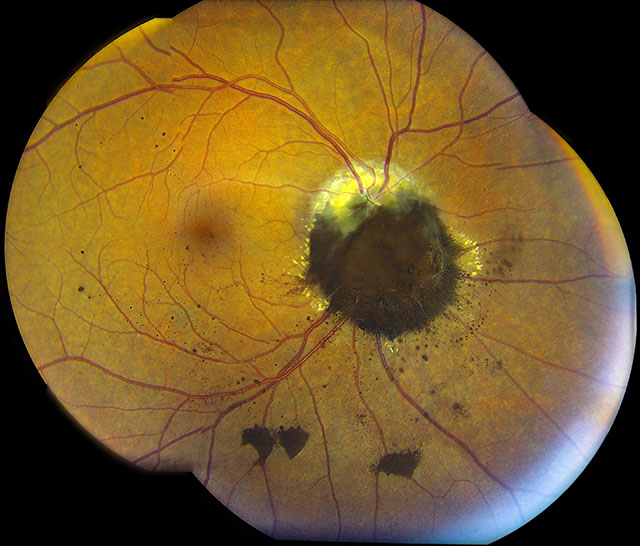
This image depicts an elevated, jet-black lesion obscuring the optic nerve, consistent with a large optic disc melanocytoma. An optic disc
melanocytoma is a benign, primary, pigmented tumor of the optic disc that is often an incidental finding during routine fundus evaluation. These
tumors are typically characterized as dark brown to black lesions, often with feathery margins.1
Echographically, these tumors display high internal acoustic reflectivity. On spectral-domain optic coherence tomography (SD-OCT), the
dense pigmentation of the lesion prevents laser penetration and results in marked shadowing. Although these lesions are usually unilateral,
bilateral cases have also been reported.2 The precise pathogenesis of
these tumors is currently unknown and there is no known association with other systemic diseases.2
Although melanocytomas do not typically grow, slow progressive enlargement has been documented in 10 to 15% of the
cases.3 Despite enlargement, true malignant transformation into melanoma
is uncommon, found in only 2% of cases.1 Pigment dispersion, like the one
in this case, has rarely been noted to accompany large optic disc melanocytomas.4-7
This is thought to occur with tumors that have invaded the retina, where free-floating cells can escape and seed into the vitreous and/or settle on
top of the retina.4
Vision is typically unaffected in eyes with optic disc melanocytomas; however, decreased visual acuity may occur in some rare cases. Causes for this may include
secondary optic neuropathy, optic disc edema, spontaneous tumor necrosis, juxtapapillary or foveal choroidal neovascularization, subretinal fluid from tumor
exudation, cystoid retinal edema, retinal traction, epiretinal membrane, central retinal vein occlusion, or, perhaps most concerning, malignant transformation
of the tumor.2,8,9
Because uveal melanoma is a main differential diagnosis, proper assessment and management of these lesions is incredibly important. Indeed, some patients have been
treated with enucleation due to confusion between melanoma and melanocytoma. Other important differentials include juxtapapillary choroidal nevus, hyperplasia of the
RPE, combined hamartoma of the retina and RPE, adenoma of the RPE, primary metastatic melanoma to optic disc, and peripapillary vitreous
hemorrhage.2 Although the diagnosis of an optic disc melanocytoma is primarily based on
clinical findings during fundus evaluation, ancillary testing may also aid in proper diagnosis. Fundus photos, autofluorescence, ultrasonography, SD-OCT, and
fluorescein angiography may support diagnosis and management.8
The majority of asymptomatic optic disc melanocytomas are managed by observation for growth and possible malignant transformation. Currently, melanocytomas that have
shown clear malignant transformation have been treated with enucleation. However, with increasing utilization of genetic testing to assess the aggressiveness of
malignant tumors, this treatment modality may change in the future.
References
1. Shields JA, Demerci H Mashayekhi A, Shields CL. Melanocytoma of Optic Disc in 115 Cases. Ophthalmology. 2004;111:1739–46.
2. Shields JA, Demirci H, Mashayekhi A, Eagle RC, Shields CL. MAJOR REVIEW Melanocytoma of the Optic Disk : A Review. 2006;51(2).
3. Joffe L, Shields JA, Osher RH, Gass JD. Clinical and follow-up studies of melanocytomas of the optic disc. Ophthalmology. 1979 Jun;86(6):1067–83.
4. Mazzuca DE Jr, Shields CL, Sinha N, Bianciotto CG, Fox G, Shields JA. Progressive retinal invasion and vitreous seeding from optic disc melanocytoma.
Clin Experiment Ophthalmol. 2012 Jan-Feb;40(1):e123-5.
5. Guo H, Li Y, Chen Z, Guo X. Vitreous seeding from a large optic disc melanocytoma. J Neuroophthalmol. 2014 Sep;34(3):276-7.
6. Al-Hinai A, Edelstein C, Burnier MN Jr. Unusual case of melanocytoma. Can J Ophthalmol. 2004 Jun;39(4):461-3.
7. Lauritzen K, Augsburger JJ, Timmes J. Vitreous seeding associated with melanocytoma of the optic disc. Retina. 1990;10(1):60-2.
8. Eldaly H, Eldaly Z. Melanocytoma of the Optic Nerve Head, Thirty-Month Follow-Up. Semin Ophthalmol. 2014 Feb 7 [Epub ahead of print]
9. Shields JA, Shields CL, Eagle RC, Singh AD, Berrocal MH, Berrocal JA. Central retinal vascular obstruction secondary to melanocytoma of the optic disc.
Arch Ophthalmol. 2001 Jan;119(1):129–33.
Aaron Gold, O.D., F.A.A.O.
ORS Fellow
|

IN THE
NEWS: Special Edition From ARVO
 |
OSPREY Trial Reports Non-Inferior Results Of RTH258 In Treatment Of Wet AMD
Repeat doses of RTH258 may effectively treat neovascular age-related macular degeneration, according to
a presentation at the Association for Research in Vision and Ophthalmology annual meeting.
Lawrence J. Singerman, MD , and colleagues conducted the OSPREY trial, a randomized, double-masked, active-controlled
study of 89 patients with AMD to evaluate the safety and efficacy of repeat doses of 6 mg of RTH258 (Alcon) vs. 2 mg of
Eylea (aflibercept, Regeneron) over 56 weeks.
In the first part of the study, the two arms had identical dosing of two 8-week treatments. At week 32, the second part
of the study included the RTH258 patients being treated every 12 weeks regardless of disease activity. Rescue treatments
for both arms were allowed at each monthly visit.
After both parts of the study, RTH258-treated patients showed visual acuity gains that were non-inferior to
aflibercept, despite a greater number of rescue treatments in the aflibercept arm.
Approximately 50% of RTH258 patients were successfully maintained with dosing every 12 weeks.
A low rate of adverse events was recorded in both arms.
Based on these results, the sponsor has initiated two global phase 3 studies, with the OSPREY trial incorporated into
the design, according to the presenter.
|
 |
No Systemic Complications Observed With Intravitreal Sirolimus For Noninfectious Uveitis
Intravitreal sirolimus does not seem to have any systemic adverse events in the treatment of noninfectious uveitis,
according to a poster presented at the Association for Research in Vision and Ophthalmology annual meeting.
Daniel Rosberger, MD, and colleagues observed the whole-blood pharmacokinetics of intravitreal sirolimus in 14 patients
who took part in the double-masked phase of the SAKURA Study 1.
In the 6-month study, three patients received intravitreal sirolimus injections of 44 µg, six received injections
of 440 µg, and five received injections of 880 µg at months 0, 2 and 4.
After the first injection, sirolimus in the blood increased, reaching a Cmax of 0.337 ng/mL, 1.97 ng/mL and 3.06 ng/mL,
at a mean Tmax of 0.69, 1.3 and 4.2 days, for those injected with 44 µg, 440 µg and 880 µg, respectively.
By day 30, sirolimus concentrations declined toward the limit of quantitation level, the study said.
The mean AUC0-60d was 1.5 ngday/mL, 15 ngday/mL and 30.5 ngday/mL, respectively.
The same pattern occurred after month 2 and month 4 injections, with no change in mean Cmax and AUC0-60d between the
first and third injections.
|
 |
Angio-OCT May Have Utility In Visualizing Choroidal Neovascularization
Angio-OCT may be a novel diagnostic technique to image patients with age-related macular degeneration or
inflammatory infections, according to a poster presented at the Association for Research in Vision and Ophthalmology
annual meeting. Vittoria Ravera, MD, and colleagues evaluated the visibility of choroidal neovascularizations in
a comparison of angio-OCT imaging, fluorescein angiography and indocyanine green angiography.
Of 20 eyes, 13 displayed occult choroidal neovascularization, four had classic choroidal neovascularization, two presented
a combination of occult and classic choroidal neovascularizations, and one had retinal angiomatous proliferation.
The choroidal neovascularization complex was visible in 99% of cases with indocyanine green angiography, but
precise characterization of the vessels from the lesion was visible in only 30% of eyes.
The choroidal neovascularization complex was visible in 100% of eyes with angio-OCT, and there was better
identification of the neovascular network, the study said.
Imaging was not limited by subretinal fluid or blood, while pigmented epithelium detachment formed mild masking in
the images, the study said.
|
 |
Ultra-Widefield Imaging Increases Diabetic Retinopathy Detection
Ultra-widefield imaging may double diabetic retinopathy detection and referral rates while decreasing the ungradable rate
in telemedicine programs, according to a a poster presented at the Association for Research in Vision and
Ophthalmology annual meeting.Mark B. Horton, MD, and colleagues evaluated the implementation of ultra-widefield imaging
of 456 patients within the Indian Health Service ocular telehealth program in comparison to 7,460 subjects imaged with
the current standard of non-mydriatic multi-field fundus photography.
Ultra-widefield imaging reduced the ungradable image rate by 85% to less than 5% per eye and by 92% to
2.1% per patient.
Diabetic retinopathy identification increased from 12% to 23%, and referable diabetic retinopathy more than
doubled from 6% to 14%.
Identification of peripheral lesions suggested more severe diabetic retinopathy severity in 20% of patients.
|
 |
Multicolor Confocal Scanning Laser Ophthalmoscopy May Effectively Detect Retinal Pathologies
Multicolor confocal scanning laser ophthalmoscopy may be combined with spectral-domain OCT to detect subretinal,
intraretinal and epiretinal pathology, according to a poster presented at the Association for Research in Vision
and Ophthalmology annual meeting. Henry Feng, MD, and colleagues discussed a retrospective review of 159 consecutive
eyes that underwent multicolor confocal scanning laser ophthalmoscopy (cSLO) with SD-OCT (Spectralis, Heidelberg) in a
single session.
Multicolor reflectance showed positive percent agreement that was equal to or greater than infrared, green and
blue reflectance images in detecting choroidal lesions, chorioretinal scars, peripapillary atrophy, drusen and
pigment epithelial detachment. It also demonstrated the highest positive percent agreement for intraretinal and
subretinal hemorrhage.
Retinal pigment epithelial atrophy was most effectively detected in infrared reflectance.
Epiretinal membrane was equally detected on green, blue, and multicolor reflectance but least detected in
infrared reflectance.
Imaging artifacts were found in 23.3% of eyes, and the study authors intend to characterize further clinical findings
in reference to the new technology, Feng said.
|
 |
Interim Results Of INJECT Study Show Real-World Jetrea Experience
The long-term effects of Jetrea may be as safe and effective as those seen in registration trials, according to a
poster presented at the Association for Research in Vision and Ophthalmology annual meeting.
Peter Stalmans, MD, PhD, and colleagues discussed the non-interventional, multicenter, prospective INJECT study of
105 patients with vitreomacular traction (VMT) treated with Jetrea (ocriplasmin, ThromboGenics).
Of the 105 patients, 64 had VMT only and 41 had VMT plus macular hole.
Ocriplasmin was ineffective in eight patients; seven patients had vitreous floaters, seven patients had photopsia,
and five patients had reduced visual acuity.
|
 |
Humira Extends Time To Treatment Failure In Noninfectious Uveitis
Humira met its primary endpoint of extending the time to treatment failure in patients with noninfectious uveitis who
still experienced intraocular inflammation despite systemic corticosteroid therapy, manufacturer AbbVie announced in a
press release at the Association for Research in Vision and Ophthalmology meeting in Denver.
The VISUAL-I study, a phase 3 multicenter, double masked.randomized, placebo-controlled study, included 217 patients
with active intermediate uveitis, posterior uveitis or panuveitis while on corticosteroid therapy.
Of the 217 patients, 110 were treated with Humira (adalimumab) and 107 received placebo.
Patients in the Humira group received an 80-mg loading dose and subsequent 40-mg subcutaneous injections every 2 weeks
for up to 80 weeks.
All patients received an initial 60-mg dose of prednisone and subsequent tapering over 15 weeks.
The primary endpoint was time to treatment failure, defined as one or more of the following in at least one eye, according
to the release: new active, inflammatory chorioretinal or vascular lesions; inability to attain an anterior cell grade
of 0.5+ or less at 6 weeks, or a two-step increase relative to best state achieved after 6 weeks; inability to attain
a vitreous haze score of 0.5+ or less at 6 weeks, or a two-step increase relative to best state achieved; a decrease in
best corrected visual acuity of at least 15 letters relative to the best state achieved.
Patients in the Humira group were significantly less likely to experience treatment failure than those in the placebo
group (P < .001). Median time to treatment failure was extended to 5.6 months in the Humira group.
The rates of adverse events were 1,047 events per 100 patient-years in the Humira group and 965 events per 100
patient-years in the placebo group. Rates of serious adverse events were 29 events per 100 patient-years in the Humira
group and 13 events per 100 patient-years in the placebo group.
Humira is a tumor necrosis factor blocker whose current therapeutic indications are for moderate to severe
rheumatoid arthritis, ankylosing spondylitis, moderate to severe plaque psoriasis, active and progressive
psoriatic arthritis, moderate to severely active Crohn's disease and moderate to severely active ulcerative colitis.
|

MEET
THE FELLOWS
In each issue, a Fellow of the Optometric Retinal Society
is highlighted. In this issue, Dr. Aaron Gold, the newest ORS fellow, will be highlighted.
After receiving a bachelor's of science degree in Microbiology and Cell Science from the University of Florida and
a bachelor's of science degree in Vision Science from Nova Southeastern University, Dr. Gold earned his Doctor of
Optometry degree from Nova Southeastern University. He then worked and taught within the ocular oncology clinic at
the world-renowned Bascom Palmer Eye Institute for several years, where he was involved in mentoring optometric students
and residents. Currently Dr. Gold is the director of optometric services at Murray Ocular Oncology & Retina, the
largest private ocular oncology practice in the southeastern United States. In addition to becoming a fellow in
the Optometric Retina Society, Dr. Gold is a fellow of the American Academy of Optometry, and a member of the
American Optometric Association, the Florida Optometric Association, the Broward County Optometric Association, and
the Miami-Dade Optometric Physicians Association. Additionally, Dr. Gold has authored multiple peer-reviewed articles
and posters in the field of ocular oncology and vitreo-retinal diseases, is a peer reviewer for multiple academic
journals, and continues to mentor optometric students and residents.
Aaron currently lives in the heart of downtown Miami, and in his spare time he enjoys exploring all the different
sights, sounds, and food that the city has to offer.
WHY
BECOME AN ORS MEMBER?
By Rex Ballinger, O.D., F.A.A.O.
Chair, Membership Committee
Membership in the Society can provide several benefits. You may
receive discounts at annual meetings. You’ll receive regular
newsletters on new and exciting updates on retinal disease diagnosis
and management as well as other newsy items of interest. And you’ll
be associated with a body of knowledge and resources which can
help you in many other ways. So consider membership in the Society.
It will be worth your while in your quest for better understanding
of the retina.
If your interests extend beyond the general, if you want to become
part of the dynamic team involved in the Society to share your
interest and enthusiasm with your colleagues, consider becoming
a Fellow member. Details and applications can be found at
www.optometricretinasociety.org |

SPONSOR NEWS

Editor
in Chief
Steven Ferrucci, OD, FAAO
Co-Editor
Mark T. Dunbar, OD, FAAO |
Journal
Reviewers
Tahrim Rahman, OD
Kristie Draskovic, OD
Savannah Brunt, OD
Nicholas Zagorianos, OD
Art/Production Director
Joe Morris
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click
here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click
here if you do not want to receive future emails from Review of Optometry. |
|