| |
Volume 14, Number 4 |
December 2018 |
|
|
Inside
This Issue |
|
|
|
|
|
This e-newsletter is provided free to doctors through industry support from |
 |
| |
FROM
THE DESK OF THE EDITOR
Happy holidays from the ORS! This time of year certainly seems to get busy. As I try to recover from the multiple, hectic Thanksgiving and Christmas celebrations, I start to reflect on what I look forward to and hope for in 2019. For me, that is the hope for good health, happiness, opportunities, and a NCAA basketball championship for my Indiana Hoosiers (ok, that might be getting too far-fetched). In the retina world, I think there is a lot to look forward to as well. I foresee continued strides in gene therapy. As new vectors and delivery mechanisms continue to develop, I hope to see more inherited retinal disease benefit from gene therapy. I think another hot area will be in artificial intelligence. Various phone apps are already in the use to measure and monitor vision. Oculocare Medical offers the Alleye app that can detect and track metamorphopsia from AMD and diabetic macular edema. A patient- run, home-based OCT system from Notal Vision may also be available as early as 2020. I look forward to many great developments over the upcoming New Year.
On another note, this newsletter marks our last message from outgoing ORS president Dr. Steve Ferrucci. I would like to take the opportunity to acknowledge Steve for his involvement within this newsletter and the ORS. Prior to me, Steve graciously served at the editor-in-chief of this newsletter for almost 10 years. Then, additionally, he supplied a president’s message over the past two years. A warm thanks to Steve for his contributions! I promise now to stop nagging you with emails and deadlines.
Happy New Year!
Anna Bedwell, OD, FAAO
Editor-in-Chief
PRESIDENT'S MESSAGE
We recently held our yearly continuing education event, ORS Retina Update 2018, in beautiful Scottsdale Ariz., Nov. 30 to Dec 1. Over 100 ODs enjoyed 11 hours of continuing education. The event began on Friday with a posterior segment technology workshop, followed by the Larry Alexander Legacy Lecture. This lecture pays respects to our dearly departed friend and mentor, Larry Alexander, who was a past president of the ORS as well as a pioneer in the optometric and optometric retina community. Saturday included a full day of lectures by esteemed ORS faculty, such as Leo Semes, Jeff Gerson, Brad Sutton, Mo Rafieetary and myself. The highlight of the day, however, was the fantastic keynote talk by Mark Barakat, MD, a retinal specialist at Retinal Consultants of Arizona, who discussed the outlook for therapies of genetic retinal disease. Overall, the meeting was a huge success. We are beginning planning for next year, so stay tuned in the coming months if you want to join us next year.
I would like to thank our partner in the meeting, Review of Optometry. Without Review, we would not be able to put on such a meeting. Special thanks to Casey, and the rest of the Review team, notably Mike, Michele, Melinda, Alex and Elizabeth.
This meeting would also not be possible without the generous support of our sponsors, both at the meeting and year round. That includes Novartis, our gold sponsor, Regeneron, our silver sponsor, and all the additional sponsors: Centervue, Diopsys, Heidelberg Engineering, Maculogix, Notal Vision, Optos, Science Based Heath, EyePromise and the International Academy of Low Vision Specialists.
Lastly, I would like to thank Mohammed Rafieetary, my education co-chair, only friend and true mastermind of the program. Always great to work with you, my friend.
And to all my readers, Happy Holidays and a healthy, prosperous 2019!!
Sincerely,
Steven Ferrucci, OD, FAAO
ORS President

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.
Answer appears later in newsletter.

Image Gallery
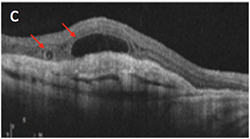
Which of the following is an OCT image of outer retinal tubulations?
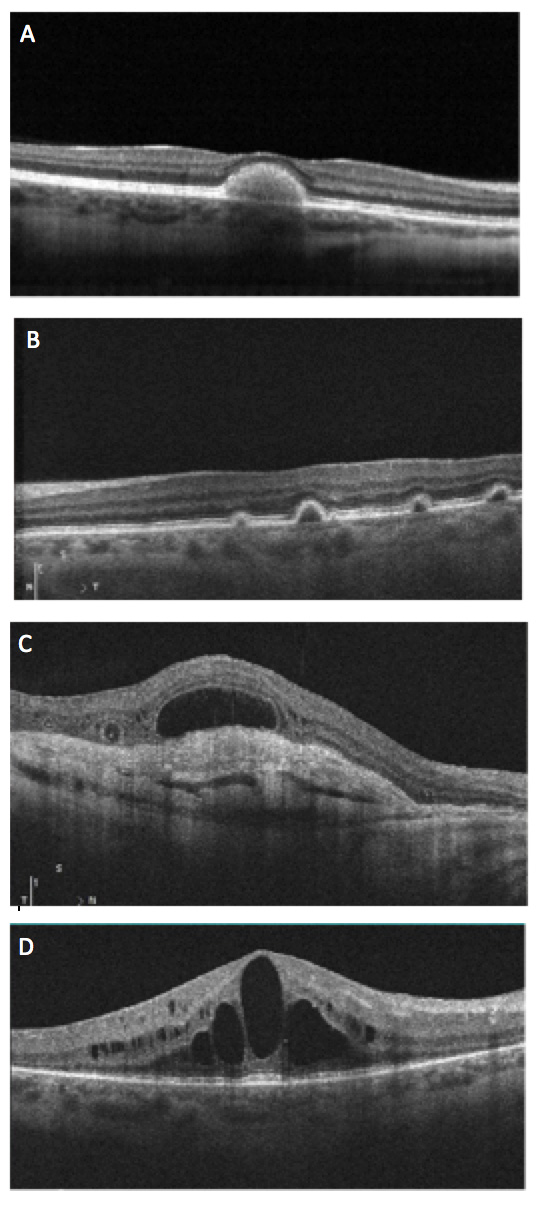
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Stroke Risk and Risk Factors in Patients with Central Retinal Artery Occlusion
As central retinal artery occlusion (CRAO) and stroke share a similar mechanism, understanding the relationship is vitally important. Questions have arisen over past years regarding proper CRAO management and whether extensive neurological and vascular workups, essentially a “stroke workup” is necessary. This particular study looked at the yield of an acute stroke workup in patients with acute CRAO.
This retrospective study looked at 103 patients seen with acute CRAO (within seven days onset of symptoms) at Vanderbilt University Medical Center between 2009 and 2017. In 2009, protocol had been established to admit all patients with acute CRAO to vascular neurology service for workup. Workup included head and neck angiography (CT or MR), MRI of the brain, echocardiography, cardiac telemetry and blood work (LDL, HgbA1c, ESR, CRP and CBC).
Of 103 patients with acute CRAO and subsequent stroke workup, there was a high yield of findings. One-third of the CRAO cohort was in hypertensive crisis, and 23% showed evidence by blood work (ESR, CRP) of serious systemic inflammatory disease. Critical carotid artery disease (ipsilateral to CRAO) was found in 36.7%. Twenty percent of CRAO patients tested on EKG had a major abnormality such as MI or heart failure. Of the 67 patients that had MRI of the brain, 37.3% showed evidence of ischemic stroke. In total, the inpatient workup resulted in 25% of CRAO patients needing an acute surgical procedure and 92% necessitating a medication change. Of the 75 patients not lost later to follow up, eight suffered incident stroke, 13 had MI and six died within two years of the CRAO event. These were higher rates than reported with TIA.
Even though this study was retrospective in nature and occurred at a single institution in an area of higher stroke rates, it still reinforces the concept that CRAO necessitates a neurological and vascular workup. The authors suggested that CRAO indicated a comparable risk for subsequent stroke/MI as a TIA or stroke and, therefore, necessitated a thorough, timely risk factor evaluation.
Lavin P, Patrylo M, Hollar M, et al. Stroke risk and risk factors in patients with central retinal artery occlusion. Am J Ophthalmol. 2018;196:96-100.
Prevalance of Cystoid Macular Oedema, Epiretinal Membrane and Cataract in Retinitis Pigmentosa
Inherited retinal disorder, retinitis pigmentosa (RP), causes progressive vision loss and carries the risk for secondary ocular complications of cystoid macular edema (CME), epiretinal membrane (ERM) and cataract. Understanding these complications is important for patient management in RP. This study sought to determine the prevalence of CME, ERM and cataract in RP patients managed at Moorfields Eye Hospital within an inherited retinal dystrophy clinic.
All patients with either RP or rod-cone dystrophy were seen within 2012 and had SD OCT and reviewable record to determine lens status. Inheritance patterns were established from pedigrees with few patients with molecular diagnosis. There were 338 eyes (of 169 patients) included with an average age of 47.1 years (±18.4). CME was detected in 50.9% of eyes, with many of the participants having bilateral CME (73.7%). CME most often occurred with younger age but had no gender predilection. It was most common in those with AD inheritance and least in X-linked. Of those with CME, 31.1% were mild enough for no treatment, 37.4% treated with oral acetazolamide and 29.3% with topical dorzolamide. ERM was observed in 22.8% of eyes and, likewise, more often bilateral in 71.7% of patients. Cataract was present in 23.4% eyes and pseudophakia in 11.2% of eyes.
Overall, this study showed a high prevalence of potentially treatable complications from RP. Futhermore, the data showed a high prevalence of bilateral eye involvement with these complications (over 70.0%). The authors thus propose the necessity to screen patients with RP using SD-OCT to detect CME and ERM.
Liew G, Strong S, Bradley P, et al. Prevalence of cystoid macular oedema, epiretinal membrane and cataract in retinitis pigmentosa. Br J Ophthalmol. 2018 Oct 5. doi: 10.1136/bjophthalmol-2018-311964. [Epub ahead of print].
Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration
A Mediterranean diet is defined by high dietary intake of plant foods and fish, low intake of meat and dairy, with olive oil fat sources and moderate wine intake. This dietary lifestyle is highly regarded for the associated lower rates of mortality and chronic disease. This study investigated the impact of a Mediterranean diet and the incidence of advanced AMD using data from two population-based prospective studies: the Rotterdam Study I (RS-I) and the Antioxydants, Lipides Essentiels, Nutrition et Maladies Oculaires (Alienor).
Overall, complete data (reliable dietary data along with follow-up information) was available on 4,996 participants (4,446 RS-I, 550 Alienor Study) without advanced AMD at baseline. Retinal photos were used to classify eyes into one of three groups: no AMD, early AMD or advanced AMD (neovascular or atrophic). Of the 4,996, 155 developed advanced AMD over follow-up. The mean follow-up time in RS-I was 9.9 years and 4.1 years in the Alienor Study. A trained dietitian interviewed each participant with a food frequency questionnaire. Participants were placed into one of three categories based on adherence to a Mediterranean diet: low, medium or high.
In both study groups, those in the high-adherence category had a lower incidence, 41% reduced risk, of development of advanced AMD. The diet as a whole was most impactful as no particular component had a significant impact. The association was higher for the development of atrophic AMD. Neovascular AMD did not show statistical significance though showed a similar association. No significant interactions were found between genetic profile (CFH or ARMS2 genes) and the dietary category.
Previous studies fall in line with results obtained in this study. This further supports an approach to prevent advanced AMD through lifestyle and dietary changes. This is a public health concern, as advanced AMD causes a significant burden to those affected and to the healthcare system.
Merle BMJ, Colijn JM, Cougnard-Grégoire A, et al. Mediterranean diet and incidence of advanced age-related macular degeneration: The EYE-RISK Consortium. Ophthalmology. 2018; Aug 13. doi: 10.1016/j.ophtha.2018.08.006. [Epub ahead of print]
Baseline Morphological Characteristics as Predictors of Final Visual Acuity in Patients with Branch Retinal Vein Occlusions: MARVEL Report No. 3
The goal of this study was to see if baseline OCTs could predict the final visual acuity outcome (one year out) in patients with macular edema due to branch retinal vein occlusions (BRVO).
This study used data from the prospective, randomized MARVEL trial with 75 patients treated with either ranibizumab or bevacizumab for BRVO. The inclusion criteria in this study included patients with center-involving macular edema caused by BRVO in the last nine months. Additionally, a minimum of 250 microns central retinal thickness (CRT) was required to be present on SD-OCT. Baseline BCVA had to be within 24 and 73 ETDRS chart letters in the affected eye or 20/40 to 20/320. Patients were excluded if they had previous treatment to the affected eye. The authors found that patients with a CRT >500 microns had greater improvement in visual acuity. Eyes with damage to the inner retina, such as GCL cystoid edema and hyper-reflective dots, had smaller gains in visual acuity while GCL cystoid edema had the highest negative predictive value. This trial also looked at the improvement in BCVA when there were abnormalities in the outer retina, the external limiting membrane and inner/outer segment line. The BCVA was found to have similar results at 12 months as it did after the second injection. While some patients needed additional injections for recurrent edema, most did not require injections in the final six months of the study.
This study concluded that 90% of the improvement in visual acuity happened by one month after the second injection. It also showed that damage to the outer retina resulted in more vision improvement following injections when compared to damage to the inner retina.
Narayanan R, Stewart MW, Chhablani J, et al. Baseline morphological characteristics as predictors of final visual acuity in patients with branch retinal vein occlusions: MARVEL report no. 3. Indian J Ophthalmol. 2018; Sep;66(9):1291-1294.
Oxygen Therapy in Patients with Retinal Artery Occlusion: A Meta-Analysis
Retinal artery occlusions, especially central retinal artery occlusions, can have a devastating impact on vision. Due to anatomical and other factors, some patients experience partial visual recovery but many do not. Various therapeutic interventions are often attempted, but there is no therapy that has proven to be consistently effective. One therapy that is often considered is the delivery of oxygen in a timely manner to perfuse the ischemic retinal tissue. This meta-analysis of seven randomized clinic trials enrolling 251 total patients evaluated the effectiveness of such an intervention. Overall, patients receiving oxygen therapy were 5.61 times more likely to experience visual improvement compared with those who did not. Specifically, the most effective therapy was 100% hyperbaric oxygen delivered for a total of nine or more hours. So what does this mean for clinicians? When a patient presents with a recent onset retinal artery occlusion, at the very least, a discussion of potential hyperbaric oxygen therapy is warranted. Given the lack of proven treatment options and the positive results found in this meta-analysis, rapid intervention with hyperbaric oxygen may indeed be the only intervention with a reasonable chance to positively influence visual recovery.
Wu X, Chen S, Li S, et al. Oxygen therapy in patients with retinal artery occlusion: A meta-analysis. PLoS One. 2018 Aug 29;13(8):e0202154.
Risk of Incident Atrial Fibrillation in Patients Presenting with Retinal Artery or Vein Occlusion: A Nationwide Cohort Study
Retinal vascular occlusions, comprised of retinal artery occlusions (RAO) and retinal vein occlusions (RVO), are significant causes of vision loss. In addition, they are often associated with serious systemic conditions. This study utilized the nationwide Danish registry to evaluate a possible association between retinal vascular occlusion and new incident atrial fibrillation (AF). Patients were excluded from the study if they had a known prior history of AF or if they had used Warfarin. A total of 1,368 patients were included, 706 with RAO and 529 with RVO. These patients were followed until they were diagnosed with AF, they died or the study period ended. New incident AF was found to be significantly associated with RAO, but not RVO. The odds ratios were 2.01 per 100 person-years for RAO, 1.52 per 100 person-years for RVO, and 1.22 per 100 person-years for a matched control group. Given these findings, patients who are diagnosed with RAO should be followed very closely for the development of AF. Being aware of this association and instituting proper management may decrease the chance of subsequent systemic ischemic stroke.
Christiansen CB, Torp-Pedersen C, Olesen JB, et al. Risk of incident atrial fibrillation in patients presenting with retinal artery or vein occlusion: a nationwide cohort study. BMC Cardiovasc Disord. 2018; May 10;18(1):91.
Central Retinal Vein Occlusion Revealing Celiac Disease: The First of Two Cases From India
Central retinal vein occlusion (CRVO) is associated with several systemic vascular disorders. This recent report from India adds another potential systemic association to consider. The authors report two young females, a 35-year-old and a 25-year-old, who both suffered non-ischemic CRVO. A comprehensive evaluation detected the presence of previously undiagnosed celiac disease in both women. Both were anemic and had significantly positive serum antitissue transglutaminase antibody titers. Anti-Ttg titers are highly sensitive and highly specific for celiac disease. The classic celiac triad of diarrhea, abdominal pain and malabsorption is fully encountered in only 10% to 20% of celiac disease patients, but all celiac patients are at increased risk for thrombosis. It is logical that patients who are at lifelong risk for thrombosis would have an elevated risk of retinal vein occlusions. When encountering patients with RVO, especially young patients, celiac disease should be considered as a potential underlying cause.
Malhi RK, Dhami A, Malhi S, et al. Central retinal vein occlusion revealing celiac disease: The first of two cases from India. Indian J Ophthalmol. 2018 Sep;66(9):1315-1317.
Cilioretinal Arteries and Collateral Vessels after Occlusion of Central Retinal Artery
Cilioretinal arteries are the most common retinal vascular anomaly found in around 10% to 50% of patients. Neovascularization is rare in central retinal artery occlusion, and has been found to occur in around 3% to 18% of these patients. The development of collaterals are thought to help spare central vision by preserving blood flow through the papillomacular region. This study retrospectively analyzed patients from the Clinic of Ophthalmology, Clinical Center in Kragujevac, Serbia from a period of January 2010 to January 2015. Ten patients were evaluated, all of whom exhibited CRAO and associated cilioretinal arteries, which were confirmed via fluorescein angiography.
The goal of this study was to observe the significance of cilioretinal arteries on the development of neovascularization and collaterals following artery occlusions. Every patient in this study presented with a cherry red spot. Additionally, five of 10 patients presented with retinal opacity and retinal artery attenuation and four of 10 patients had optic nerve head edema. Visual acuity was decreased one year after the CRAO compared to initial examination. Patients who had multiple cilioretinal arteries and the three of 10 who formed collaterals were found to have decreased visual acuity after the initial examination. This led to the conclusion that the retina atrophy was not preserved by the presence of cilioretinal arteries or collaterals. Of the 10 patients in this study, three developed neovascularization.
The interpretation of this study was that unfortunately those who had one or more cilioretinal arteries and pre-existing or new developed collaterals did not benefit long-term from a visual acuity standpoint. The presence of collaterals and any cilioretinal arteries were not able to prevent the visual acuity from decreasing or neovascularization from developing.
Petrović N, Šarenac T, Todorović M, et al. Cilioretinal arteries and collateral vessels after occlusion of central retinal artery. Vojnosanit Pregl 2018; 75(7):716–720.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
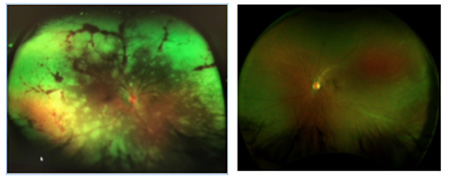
A 41-year-old-Hispanic male presented to the emergency room (ER) due to severe coughing, headaches, chills, fever and pressure around the chest. The ER diagnosed him with bronchitis. He was prescribed acetaminophen and NSAIDs and advised to rest and drink plenty of fluids. At the time, he mentioned that his right eye was a slightly red, but the ER presumed the redness was a result of the coughing and assured quick recovery. Within two days, he noticed increased pain and a decrease in peripheral and central vision. He returned to the ER and was referred to the on-call ophthalmologist, and upon inspection of the retina (photos above), he was immediately admitted. Labs and imaging came back unremarkable but the retinal findings were highly suspicious of a viral infection, and the patient was put on IV antivirals. One week later, there was no progression in necrosis but the ophthalmologist noticed an impending retinal detachment and, therefore, performed retinal repair. During the surgery, the surgeon noticed an active infection in the left eye and immediately administered antivirals and steroids. Regression followed within two weeks, and the patient was diagnosed with acute retinal necrosis (ARN) secondary to shingles.
Six months later, the patient presented for a low-vision exam with the following findings: Visual acuities were LP OD and 20/20 OS. Pupils were round and equal but the right eye had a sluggish reaction and a positive APD. There was complete field restriction in the right eye. His intraocular pressure measured 18 mmHg OD and 16 mmHg OS. Slit-lamp examination in the right eye was remarkable for corneal keratic precipitates and an opaque cataract, preventing retinal view. In the left eye, the posterior segment had areas of necrosis in the periphery but no signs of an active virus. The plan was to fit him with Peli prisms and send a referral for occupational therapy.
Top differential diagnoses when presenting with necrosis of the retina are progressive outer retinal necrosis, cytomegalovirus retinitis, toxoplasma chorioretinitis, ocular syphilis, sarcoidosis, intraocular lymphoma, Eales disease and systemic lupus erythematosus. The American Uveitis Society established the following as diagnostic criteria in 1994 for ARN: one or more foci of retinal necrosis with discrete borders located in the peripheral retina, with rapid progression in absence of antiviral therapy, circumferential spread, occlusive vasculopathy affecting arterioles and prominent vitritis and/or anterior chamber inflammation.1 Oftentimes, a vitreous tap can also help with diagnosis.
ARN generally affects otherwise healthy, immunocompetent adults. The etiology of the infection is more commonly due to varicella-zoster virus (VZV) and less commonly due to herpes simplex virus. VZV remains dormant in ganglion cells and is triggered by stress, lack of sleep, weakened immune system, fever and trauma.2 There may be a genetic risk association in patients who have the HLA-DQw7 antigen and the HLA-Bw62, DR4 phenotype.3 Patients at risk could have a subtype of deficiency in their immune systems. The virus usually migrates from the trigeminal ganglion to the eyes inside the retina, causing severe inflammatory response. The retinal arterioles become inflamed resulting in vaso-occlusion and rapid necrosis of retinal tissue. The other eye can also be affected through retrograde axonal transport. Due to thinned necrotic retina, 50% to 75% of ARN cases develop a retinal detachment.2 Patients’ symptoms include redness, photophobia, pain, floaters and progressive vision loss in one eye. Clinical signs include episcleritis, keratic precipitates, anterior chamber reaction, vitreous inflammation, peripheral retinitis, retinal vascular occlusions and patches of multifocal areas of retinal whitening. No control studies on treatment have been done due to the rarity of ARN. First line of treatment includes antivirals, delivered either orally, intravenously and/or intravitreally. Anti-inflammatory and antithrombotic therapy may also be considered. Laser photocoagulation may be done prophylactically to avoid retinal detachment. A retrospective study showed that a combination of intravitreal and systemic treatment was better than systemic therapy alone.4 Also, the use of steroids can be beneficial after starting antiviral therapy and not before.5
Prognosis usually depends on vision at the time of presentation. Other factors that can affect the prognosis is whether the individual has secondary retinal detachment or ischemic optic neuropathy. Any ARN suspect needs immediate medical attention. More research is needed to better understand the onset of ARN and who may be susceptible to acquiring the disease.
Steven Kim, OD
Primary Eye Care Resident
Brooke Army Medical Center
University of the Incarnate Word
1. Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. American Journal of Ophthalmology. 1994;117(5):663-7.
2. Holland G, Cornell P, et al. an association between acute retinal necrosis syndrome and HLA-DQw7 and Phenotype Bw62, DR4. American Journal of Ophthalmology. 1989; 108(4):370-374.
3. Schoenberger SD, Kim SJ, Thorne JE, et al. Diagnosis and treatment of acute retinal necrosis: A report by the American Academy of Ophthalmology. Ophthalmology. 2017;124(3):382-92.
4. Flaxel C, et al. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome. Trans Am Ophthalmology Society 2013; 111: 133-144.
5. Choudhury H, et al. Intravitreal triamcinolone acetonide as an adjuvant in the management of acute retinal necrosis. Can J Ophthalmology. 2014 Jun;49(3): 279-82.
|

IN THE
NEWS
 |
EyePoint Pharmaceutical’s YUTIQ™ Receives FDA Approval
EyePoint Pharmaceuticals announced that the FDA granted approval to YUTIQ™, a fluocinolone acetonide intravitreal implant, for the treatment of chronic non-infectious posterior segment uveitis. YUTIQ™ is delivered utilizing EyePoint’s Duraset™ technology. The system releases 0.18 mg fluocinolone acetonide over 36 months via a non-bioerodible intravitreal mico-insert. In Phase III clinical trials, YUTIQ™ met the primary endpoint of reducing flare-ups of uveitis.
EyePoint also has planned a next-generation, shorter-duration version of YUTIQ™. This will offer a shorter delivery period, giving more dosing flexibility for practitioners. According to press release, the company intends to file application for approval of this new insert in 2019.
|
 |
Diopsys Releases Screening Test to Detect Retinal Dysfunction Caused by Diabetes
Diopsys announced the release of the Chromatic Flash Vision Screener, an electrophysiologic screening protocol made to identify early retinal dysfunction caused by diabetes. The in-office screening test utilizes a handheld mini-Ganzfeld to present a red-on-blue flash ERG to determine electrical potential in the retina. By comparing results to a healthy, normative database, clinicians can assess the potential for development of diabetic retinopathy. The screening test is available within the Diopsys® Full Field Electroretinography module.
|
 |
Two-year Data Announced for Novatis Pharmaceutical’s Brolucizumab
Novartis has announced positive two-year results within its brolucizumab Phase III studies, HAWK and HARRIER. At two-year end (96 weeks), secondary endpoints showed superiority over aflibercept for brolucizumab 6 mg to decrease retinal fluid in patients with neovascular AMD. Similar to year one data, brolucizumab 6 mg confirmed better reduction in central subfield thickness versus aflibercept. Visual acuity gains obtained in year one were maintained. Brolucizumab (RTH258) is a humanized, single-chain antibody fragment (scFv) with high affinity and inhibition of all VEGF-A isoforms.
|
 |
FDA Approves ACTPen™ for Treatment of Giant Cell Arteritis
Genentech’s Actemra® (tocilizumab) was approved by the FDA as a single-dose prefilled autoinjector called ACTPen™ 162 mg/0.9 mL for the treatment of giant cell arteritis and adults with moderate to severe rheumatoid arthritis with inadequate response to disease-modifying anti-rheumatic drugs. The ACTPen is also approved for children ages two and up with active polyarticular juvenile idiopathic arthritis (PJIA) or active systemic juvenile idiopathic arthritis (SJIA) when administered by caregiver. Genentech expects ACTPen to be available in January 2019.
|
 |
FDA Grants Breakthrough Device Designation for Notal’s Home-based OCT
Notal Vision announced that the FDA has given Breakthrough Device designation to its product, a home-based OCT system. This designation, designed to accelerate patient access to new technologies, shows that the FDA plans to cooperate and communicate with the company as the device develops and goes through review. Notal Vision relayed through its indication for use statement that the device is "an Artificial Intelligence (AI)-based Home Use device indicated for automated identification of intra- and/or subretinal fluid in the central 10 degrees of eyes diagnosed with exudative age-related macular degeneration (eAMD). The Notal Home OCT device is intended for testing at home between regularly scheduled clinic assessments and not intended to replace standard-of-care regularly scheduled examinations and clinical testing by an ophthalmic retinal specialist."
|
| |
|

|
IMAGE QUIZ ANSWER
The correct answer is C. Outer retinal tubulations (ORT) are an OCT finding observed in a vast variety of retinal conditions, seen generally, when there is significant retinal damage. ORTs theoretically occur as the photoreceptor layer folds outward to form the tubule shape. No treatment is needed. More importantly, ORTs must be correctly identified as to not mistake for fluid or cystic edema. ORTs have a hyperflective edge, as noted in this image by the arrows, which helps to better differentiate the finding.
A: Best’s dystrophy B: Multiple idiopathic PED syndrome D: CME in RP
CORRECTION: The answers in the image gallery in the September ORS newsletter were incorrectly noted. Image B was Stargardt’s Dystrophy and image C represented Cone-rod Dystrophy.
|
MEET THE FELLOWS
Carolyn Majcher is a doctor of optometry and a fellow of the American Academy of Optometry.
She received her Doctorate of Optometry from the Pennsylvania College of Optometry at Salus University and completed an ocular disease residency at the Eye Institute of the Pennsylvania College of Optometry. Following completion of her residency, Dr. Majcher became and remains a full-time faculty member at the University of the Incarnate Word Rosenberg School of Optometry where she is an assistant clinical professor and chief of the retinal disease clinic. She is an instructor within the posterior segment disease, glaucoma and neuro-ophthalmic disease courses. Dr. Majcher’s research interests include retinal imaging and OCT angiography.
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
SPONSOR NEWS

Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Daniel Bollier, OD
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|