| |
Volume 17, Number 4 |
December 2021 |
|
FROM
THE DESK OF THE EDITOR
Seasons greetings to our readers! The holiday season is reaching its peak. Unfortunately, that is coinciding with a new COVID-19 variant, Omicron, rapidly accelerating. A few days ago, I read that my home state, Indiana, ranks as the least safe state in the country during the pandemic. This was based on rates of vaccinations, COVID-19 deaths, hospitalizations, transmission rate and positive testing rate. I always take pride in being a Hoosier so this was a blow to the gut. My New Year’s resolution for 2021 was to have a positive attitude and outlook, which was going pretty good in the earlier half of the year... Now, I can certainly say that I’m ready for 2022 to start. I have no clue what it will bring. I doubt much will change as to how we operate in patient care. I fear we may be in store for more staffing issues with doctors and employees out sick. So at least for 2022, I think I’m going to head back to my pessimistic, glass-half-empty outlook.
Fortunately, my outlook on optometric and retinal care is much more positive. As clinical trial results come through my inbox, I know there is a lot to look forward to in the years to come especially in new treatments for AMD, DME and gene therapy. So please enjoy our content below including Dr. Rafieetary’s summary of our annual meeting. Hoping you all have happy, safe (maybe steer clear of Indiana) holiday celebrations!
Cheers!
Anna Bedwell, OD, FAAO, FORS
Editor-in-Chief
PRESIDENT'S MESSAGE
It is hard to believe the end of 2021 has approached so fast. We just had the Optometric Retina Society’s annual Retina Update meeting in association with Medscape Live and Review Education Group. The meeting once again was a great success thanks to all the organizers, sponsors and most importantly, the nearly 60 live and over 300 virtual audience. Earlier this year, due to uncertainty about day-to-day COVID-related circumstances and other logistical considerations, the decision was made to coincide our meeting with the Optometric Glaucoma Symposium. The Glaucoma Symposium meeting was on Friday to noon Saturday, followed by the ORS meeting until late afternoon Sunday. Many audience members expressed their gratitude for this change, since these meetings previously were on the first two consecutive weekends of December, so many people had to pick and choose. We will soon start planning for the 2022 meeting.
We also conducted a business meeting during the event. This was offered on a virtual platform for those fellows that could not attend the meeting. The main theme of the business meeting was finding ways to help to give ORS more exposure—by involving the Posterior Segment educator, and offering schools and colleges a different path to fellowship to promote the organization to the students and residents.
I am also pleased to announce the featured article about ORS in the Nov-Dec issue of Modern Optometry and urge you to look it up.
Wishing you a wonderful and safe holiday season.
Mohammad Rafieetary, OD, FAAO, FORS
President, Optometric Retina Society

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.

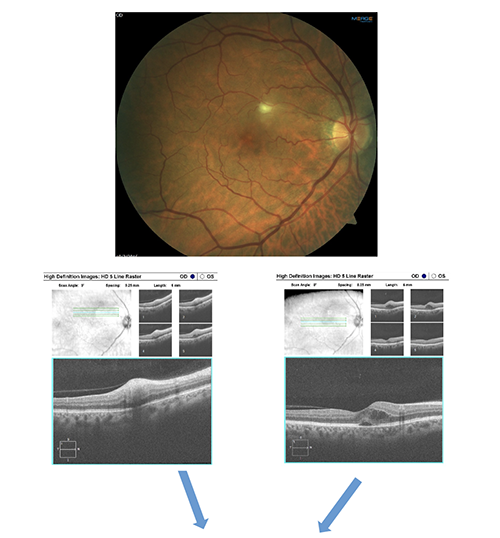
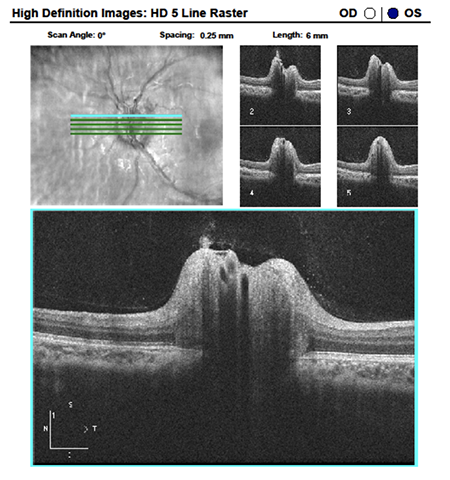
Image Gallery
What do the highlighed structures below represent?
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Pneumatic Vitreolysis with Perfluoropropane for Vitreomacular Traction with and without Macular Hole: DRCR Retina Network Protocols AG and AH
DRCR network designed a randomized clinical trial to see if pneumatic vitreolysis (PVL) could offer a safe, low-cost treatment option for vitreomacular traction (VMT, protocol AG) and macular hole (MH, protocol AH). In protocol AG, there were 46 participants (out of the recruitment goal of 124) with symptomatic VMT and an adhesion of 3000 μm or less and a VA in the range of 20/32 to 20/400. Of those, 24 were assigned to the injection 0.3 mL of C3F8 gas and 22 to sham injection. AT 24 weeks, 18/23 (78%) of the treatment group that completed follow-up had a release of VMT without need for a rescue vitrectomy. In the sham group, 2/22 (9%) had a release of VMT. Rescue vitrectomy was necessary in 1/23 in the PVL group due to development of MH. Two eyes in the PVL group developed retinal detachment and had subsequent vitrectomy.
To be included in protocol AH, participants had central VMT with a full thickness MH of 250 μm width at the narrowest point and an area of adhesion less than 3000 μm. The VA ranged from 20/25 to 20/400. There was only a treatment arm in which 35 received PVL. Participants had to position face down for 50% of the time for at least 4 days after treatment. At 8 weeks post-PVL, MH closure occurred in 10/35 eyes (29%) and 12 eyes (34%) had a rescue vitrectomy. At 24-week study completion, the number of eyes that received rescue vitrectomy increased to 23 of 34. Across the two protocols, 7/59 (12%) eyes that received PVL developed retinal tear or detachment prompting the study to be terminated. Even though the study was terminated early, there were significant take-home points. The study showed that PVL was very effective in releasing VMT in AG. The closure rate in MH was far lower at 29% especially in comparison to the high success rates of vitrectomy. Across both protocols, but more so in AH, there was a significant number of retinal detachments and tears, raising concern for safety. It is unknown whether modifications to the injection procedure or considering an alternative gas would reduce the rate of complication.
Chan CK, Mein CE, Glassman AR, et al; DRCR Retina Network. Pneumatic vitreolysis with perfluoropropane for vitreomacular traction with and without macular hole: DRCR Retina Network Protocols AG and AH. Ophthalmology. 2021; May 12. [Epub ahead of print].
Complications of Acute Posterior Vitreous Detachment
Acute posterior vitreous detachment (PVD) commonly presents in optometric practices. While the majority of PVDs occur uncomplicated, there is the risk of retinal tear (RT) or rhegmatogenous retinal detachment (RRD) occurrence. This study evaluated the risk factors for RT or RRD in the setting of an acute symptomatic PVD. In this retrospective cohort study, the medical records of a large healthcare setting, Kaiser Permanente Northern California, were queried to identify patients with acute PVD in the 2018 calendar to identify patients with a new (<90 days of symptoms) symptomatic PVD. Patients were excluded if they had less than one year of subsequent KPNC membership. After chart review, the cohort was narrowed to 8305 patients.
The average age at acute PVD presentation was 63.8 years, with more women than men (61.2% vs. 38.8%). Almost all (94.6%) complained of floaters and about half (44.9%%) had symptoms of flashes. Most were examined by comprehensive ophthalmologists (83.6%) and the rest by optometrists (16.4%). At initial presentation, the complication rate for acute PVD was RT in 5.4% and RRD in 4.0%. The study group did two separate multivariate analyses. The first was to consider triage or screening of patients for risk factors for complication. The predictors that indicated a significantly higher risk for complication included blurred vision, male sex, family history of RD, age younger than 60, prior cataract or keratorefractive surgery and symptom duration of one week or less. Flashes were found to be protective against complication. The second analysis was of examination findings associated with higher risk for complication. This included vitreous pigment, vitreous hemorrhage, lattice degeneration, retinal hemorrhage, and VA worse than 20/40. Refractive information was known for 7362 patients. Myopia was associated with increased risk while hyperopia (>+1.00D) was protective. The authors also analyzed for late events (within one year of PVD diagnosis). Only 1.8% (133/7522) of the initially uncomplicated PVDs developed a late complication of RT or RRD. For these, the average time between the index visit and complication was 58 days. Most predictive for a late complication was vitreous hemorrhage, followed by lattice degeneration then a history of RT or RD in the fellow eye. Those with one of these three risk factors had a 12.4% rate of late RT or RRD (vs. 0.7% risk with none).
One of the strongest attributes of this study is that the data was collected in a primary eye care setting rather than in a retinal specialty service, which likely contributed to the lower rate of complication (9.4%) as compared to previous studies (15-27%). The study also emphasized risk factors, which can be used for screening or triage and in examination to alert a clinician for higher risk.
Seider MI, Conell C, Melles RB. Complications of acute posterior vitreous detachment. Ophthalmology. 2021; Jul 27. [Epub ahead of print].
Central Retinal Vein Occlusion in Young Patients: Clinical Characteristics and Prognostic Factors
Central retinal vein occlusion (CRVO) is not common in patients under 50, but it does occur in that age group. This study from Korea retrospectively looked at CRVO patients who had reported to a large medical center over the course of six years and compared the characteristics of patients under 50 to those of patients over 50. It included 69 patients (26.2%) under age 50 and 194 patients over age 50. Compared to older patients with CVRO, younger CRVO patients had better visual acuity, less macular edema, and were less likely to have ischemic CRVO. The younger patients were less likely to have an APD, had a shorter duration of symptoms before presenting for care, and had better final visual acuity. Younger CRVO patients were more likely to be heterozygous for Factor V Leiden, but more common cardiovascular diseases such as hypertension were equally common in the younger and the older group. While younger CRVO patients had better visual outcomes in general, there were found to be some factors that predisposed younger patients to poor final visual acuity. These factors included male sex, renal disease, poor baseline visual acuity, and older age within the young age group.
Eah KS, Kim YN, Park YJ, et al. Central retinal vein occlusion in young patients: clinical characteristics and prognostic factors. Retina. 2021; Mar 1;41(3):630-7.
Outcomes of Eyes Lost to Follow-Up in Patients with Central Retinal Vein Occlusion Who are Receiving Anti-Vascular Endothelial Growth Factor Treatment
In the management of central retinal vein occlusion (CRVO) with anti-VEGF injections, the results obtained in tightly controlled clinical trials are often superior to outcomes in the real world. One of the major reasons for this involves patients lost to follow-up (LTFU) for extended periods of time. Studies have shown that up to 25% of retinal vein occlusion patients undergoing anti-VEGF therapy are lost to follow-up for more than 12 months. This retrospective study from China examined the visual consequences of CRVO patients LTFU for a period of six months or more. Patients were excluded if they were known to have ischemic CRVO before being LTFU, had any neovascular complications before being LTFU, had diabetes, had other retinal conditions, had a history of prior retinal treatment before the CRVO, or had glaucoma/ocular hypertension. In total, a relatively small number of patients met the criteria, with 17 total patients included. Prior to becoming LTFU, the mean BCVA of these 17 patients was 20/79 Snellen equivalent, and the mean central foveal thickness (CFT) was 360.9 microns. The most common reasons for becoming lost to follow-up were noncompliance, financial issues, and illness. Before becoming LTFU, patients had been treated for an average of 6.1 months and had received an average of 4.5 injections. After becoming LTFU for an average of 7.8 months, the average BCVA at the first return visit had dropped to a Snellen equivalent of 20/693. In addition, 7 of the 17 eyes had developed neovascular complications. Mean central retinal thickness had increased to 738.7 microns. Upon returning after being lost to follow-up, all eyes underwent further treatment via various modalities as appropriate, including anti-VEGF injections, steroid implants, pan-retinal laser, and vitrectomy. At the final follow up visit, the average Snellen equivalent BCVA was 20/364, and the average CCT was 469.9 microns. While some gain in vision was accomplished with treatment upon returning, there was still considerable loss compared to the baseline established before being lost to follow-up.
Yang KB, Liu L, Feng H, et al. Outcomes of eyes lost to follow-up in patients with central retinal vein occlusion who are receiving anti-vascular endothelial growth factor treatment. Ther Clin Risk Manag. 2021; May 26;17:489-496.
Central and Branch Retinal Artery Occlusion – Do They Harbor the Same Risk of Further Ischemic Events?
Previous studies have shown an increased risk of stroke following a retinal artery occlusion; however, studies have not determined if the risk of cardiovascular events differs between central retinal artery occlusion (CRAO) and branch retinal artery occlusion (BRAO). The aim of this study was to determine if rates of ischemic stroke, myocardial infarction, and all-cause mortality differed among the two groups.
An 11-year-long single-center, retrospective study included 131 patients with confirmed CRAO or BRAO by ophthalmic examination. Researchers found no significant difference between CRAO and BRAO in frequency of subsequent ischemic stroke, myocardial infarction, or all-cause mortality. This remained true for patients with and without previous cardiovascular events. Frequency of ischemic stroke following artery occlusion was 9.9%, myocardial infarction was 2.3%, and all-cause mortality was 22.9%. Myocardial infarctions showed greater incidence before a retinal artery occlusion than after, while ischemic stroke showed no significant difference in incidence before or after a retinal artery occlusion. Cardiovascular risk factors including diabetes mellitus, hypertension, dyslipidemia, smoking, obesity, and renal disease were similar between CRAO and BRAO groups. The only significant difference between groups was increased triglyceride levels in the BRAO group.
This study showed a similar incidence of cardiovascular events occurring status post-CRAO and -BRAO. Thus, it is important to assess the risk of recurrent cardiovascular complications after both a CRAO and a BRAO.
Roskal-Wałek J, Wałek P, Biskup M, et al. Central and branch retinal artery occlusion-do they harbor the same risk of further ischemic events? J Clin Med. 2021; Jul 13;10(14):3093.
Correlation Between the Nonperfusion Area on Ultra-Widefield Fluorescein Angiography and Nonflow area of Optical Coherence Tomographic Angiography in Retinal Vein Occlusion
A retinal vein occlusion (RVO) is thought to be caused by compression of a retinal vein by a retinal arteriole. The RVO causes ischemia and the release of vascular endothelial growth factor (VEGF), which leads to an increase in non-perfusion and worsening ischemia. This ischemia is found in both the macula and in the peripheral retina and the risk of these patients developing retinal neovascularization is linked to the degree of retinal non-perfusion.
The aim of this cross-sectional study was to describe the correlation between the areas of non-perfused peripheral retina and nonflow areas of the macula in RVO patients. This was done by comparing ultra-widefield fluorescein angiography (UWFFA) to optical coherence tomographic angiography (OCTA) in 46 eyes of 46 RVO patients (21 BRVO and 25 CRVO eyes). Even though the OCTA field of view is significantly smaller than the widefield view, researchers found a statistically significant correlation between the non-perfused areas (NPA) on UWFFA and the nonflow areas on 3x3 mm OCTA (r value 0.688, p<0.01). Additionally, the ischemic index (ISI) on UWFFA significantly correlated with the NFA on OCTA in RVO (r value 0.680, p<0.01). Other OCTA parameters, including vessel density in the superficial and deep capillary plexi and the foveal avascular zone area, did not have a correlation to the NPA of UWFFA.
The findings suggested that peripheral retinal areas of non-perfusion were correlated with the nonflow macular areas. Detecting these central nonflow areas on OCTA in RVO patients may be an indicator of overall ischemic status.
Huang J, Lu Y, Gu X, et al. Correlation between the nonperfusion area on ultra-widefield fluorescein angiography and nonflow area on optical coherence tomographic angiography in retinal vein occlusion. J Ophthalmol. 2021;2021:5581319.
Vitrectomy for Diabetic Macular Edema and the Relevance of External Limiting Membrane
Diabetic macular edema (DME) is a common retinal complication occurring from pericyte loss, blood retinal barrier breakdown, and endothelial cell junction breakdown. DME is characterized as an accumulation of intra- and eventually subretinal fluid in the macula. Intravitreal anti-VEGF or corticosteroid injections are common first-line treatments in cases of DME, but OCT structural biomarkers may be helpful in guiding clinicians toward other treatment options. The aim of this retrospective study was to review DME patients who also underwent a pars plana vitrectomy (PPV) and evaluate if their external limiting membrane (ELM) configuration had an impact on their visual or morphological outcome. Nineteen eyes of 17 patients (intact ELM n=8, disrupted ELM n=11) were reviewed for this study, and all eyes underwent a PPV with combined epiretinal membrane (ERM) and internal limiting membrane (ILM) peel. Best-corrected visual acuity (BCVA) and central macular thickness (CMT) were the main outcome measures used. CMT improved across both groups. Those with an intact ELM had a better post-operative improvement in visual acuity 9 (0.28 ± 0.14 vs. 0.7 ± 0.25 logMAR, p=0.01).
The study demonstrated that DME eyes with an intact ELM prior to surgery had greater improvement in final visual acuity and less preoperative swelling compared to eyes that had ELM disruption. Of note, the study was limited in its low sample size and retrospective design. Further study is necessary to explore the ELM as a biomarker for early PPV in DME.
Ivastinovic D, Haas A, Weger M, et al. Vitrectomy for diabetic macular edema and the relevance of external limiting membrane. BMC Ophthalmol. 2021; Sep 15;21(1):334.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
Cat Scratch Retinitis
A sixty-five-year-old Caucasian male presented complaining of a new brown spot just below his line of sight in the right eye that had begun two days prior. His health history was significant for high cholesterol and hypertension for which he took Lipitor and lisinopril. Ocular history was unremarkable. The patient lived on a farm as a horse breeder.
Best-corrected visual acuities were 20/40 right eye and 20/20 left eye. Preliminary testing was normal. Anterior segment exam in both eyes was unremarkable including a quiet anterior chamber. On posterior segment exam, the right eye showed trace vitreal cells and a hazy white retinal infiltrate superior to the fovea. On OCT, the infiltrate was superficially located in the inner retina. Also present was macular edema and a small neurosensory detachment at the fovea. Fundus exam in the left eye was normal aside from a mid-peripheral CHRPE of 1.5-disc area in size.
The retinal findings in the right eye were concerning for infectious retinitis. Further discussion into the patient’s social history revealed that he had many cats and a few kittens that were a few months old. He noted frequent scratches from the kittens, leading to a high clinical suspicion for cat scratch disease (CSD). Labs were ordered including CBC, ESR, FTA-ABS, ACE, and antibody titers for toxoplasmosis, bartonella and lyme. The patient was set up with an appointment with a uveitis specialist. In the meantime, he was started on Bactrim DS q12h. The lab testing came back positive with elevated IgG antibodies to Bartonella henselae. The rest of the lab testing came back normal. The uveitis specialist agreed with a diagnosis of cat scratch retinitis and recommended switching treatment to azithromycin. The retinal findings fully resolved over the course of a few weeks and the patient’s visual acuity improved to 20/20 in the right eye.
In humans, CSD is acquired through a scratch, lick or bite from a cat or kitten infected with Bartonella, a gram-negative bacteria. Systemic symptoms of CSD include fever, malaise and lymphadenopathy. Approximately 5-10% of those with CSD go on to develop ocular manifestations generally 1-2 weeks after the systemic symptoms have resolved. These can include Parinaud’s oculoglandular syndrome, neuroretinitis, multifocal retinitis, uveitis and vascular occlusions. The most common intraocular and “classic” manifestation of CSD is neuroretinitis, inflammation of the optic nerve with macular star exudate. However, more recent case reports note that inner retinal and chorioretinal lesions may be more common in CSD, similar to this patient’s clinical presentation. Findings are most often unilateral, though bilateral presentation does occur. Lab testing for Bartonella henselae IgG and IgM antibodies can further aid in proper diagnosis. Though indirect fluorescence assay (IFA) testing and enzyme-linked immunoassay (ELISA) can show variable sensitivity and specificity.
The course for CSD systemically and for ocular manifestations tends to be self-limiting. Oral antibiotics can be utilized, especially for severe disease, but there is no clear consensus on the antibiotic of choice and duration of treatment for ocular CSD. Case reports suggest oral azithromycin, doxycycline or rifampin and may be used in conjunction with oral or topical steroids.
Anna Bedwell, OD, FAAO, FORS
E
ditor-in-Chief
References:
Chi SL, Stinnett S, Eggenberger E, et al. Clinical characteristics in 53 patients with cat scratch optic neuropathy. Ophthalmology. 2012; Jan;119(1):183-7.
Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018; Jun;96(4):e524-32.
Johnson A. Ocular complications of cat scratch disease. Br J Ophthalmol. 2020; Dec;104(12):1640-6.
Kalogeropoulos, D., Asproudis, I., Stefaniotou, M. et al. Bartonella henselae- and quintana-associated uveitis: a case series and approach of a potentially severe disease with a broad spectrum of ocular manifestations. Int Ophthalmol. 2019; 39: 2505–15.
|

IN THE
NEWS
|
 FDA Approves Port Delivery Implant for Neovascular AMD FDA Approves Port Delivery Implant for Neovascular AMD
Susvimo (Genentech/Roche), a port delivery implant, received approval from the FDA for the treatment of wet AMD. Susvimo is the first of its kind to offer an alternative to injections with the potential to significantly reduce the treatment burden for patients with wet AMD. The implant delivers ranibizumab continuously, needing as few as two refills per year. Susvimo is surgically implanted at the pars plana in a one-time, outpatient procedure. The ARCHWAY trial showed that the implant was generally well-tolerated. However, the trial did find a three-fold higher rate of endophthalmitis than the control arm of monthly ranibizumab injections.
|
|
 Patient Dosing Started in Oculis Phase 3 Study for Topical Treatment of DME Patient Dosing Started in Oculis Phase 3 Study for Topical Treatment of DME
Oculis has launched its Phase 3 DIAMOND (DIAbetic Macular edema patients ON a Drop) study, announcing in November that the first patients have been dosed. The drop, OCS-01, is a high concentration, preservative-free formulation of dexamethasone to be used in the treatment of diabetic macular edema (DME). If successful, this would offer a noninvasive treatment that would not require specialist management; thus, the potential to reduce burden to the health care system. Phase 2b (144 patient) results were promising by showing an improvement in best-corrected visual acuity and reduction of central macular thickness. The phase 3 DIAMOND is multicentered across multiple countries and is doubled-masked, randomized with a placebo vehicle control.
|
|
 LumiThera Acquires Diopsys LumiThera Acquires Diopsys
LumiThera, a commercial-stage medical device company known for photobiomodulation (PBM), announced entry into a definitive merger agreement with Diopsys. In the agreement, approved by stockholders of both parties, Diopsys will become a wholly owned subsidiary of LumiThera. As a leader in visual electrophysiology medical devices, Diopsys offers both VEP and ERG vision testing technology. The acquisition is expected to deliver a complementary diagnostic and treatment monitoring capabilities to LumiThera’s existing PBM platform. |
|
 Foundation Fighting Blindness and Nixon Visions Foundation Team Up on IRDs Foundation Fighting Blindness and Nixon Visions Foundation Team Up on IRDs
The Foundation Fighting Blindness announced the development of the Nixon Visions Foundation Inherited Macular Dystrophy Program, a collaborative effort between the two organizations. Over the course of three years, the program will fund six early translational research projects specific to treatment of inherited macular dystrophy with an emphasis on variants in the PRPH2 gene.
|
| |
 Haag-Streit to Focus Portfolio on Ophthalmology Haag-Streit to Focus Portfolio on Ophthalmology
Haag-Streit announced a reduction in its surgical portfolio that will allow for increased focus on diagnostic and surgical microscopes in ophthalmology. The company plans to discontinue products in neurosurgery, spine surgery, ENT and plastic-reconstructive surgery. According to a company press release, to continue meeting the highest standards, surgical microscopes will be developed and manufactured in close cooperation between Germany and Switzerland.
|
|
|

|
IMAGE QUIZ ANSWER
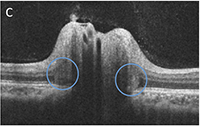
Answer C is the correct response. PHOMS, which stands for peripapillary hyperreflective ovid mass-like structure, is an OCT finding best noted with SD-OCT using enhanced depth imaging. PHOMS represent herniated optic nerve fibers bulging into the peripapillary region in discs with axoplasmic stasis. PHOMS wrap around the nerve in a centrifugal fashion, like the shape of a donut. On an OCT cross-sectional slice, they are diffusely hyper-reflective and always adjacent to the disc margin just above Bruch’s membrane. PHOMES have been reported in many conditions that lead to axoplasmic stasis including papilledema, optic disc drusen, NAION and tilted discs. When present in conjunction with a swollen disc, PHOMS will disappear as the disc edema resolves.
A: blood vessels B: optic nerve drusen
D: scleral crescents
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Jenna Wellen, OD
Brooke Tobias, OD
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|