| |
Volume 13, Number 1 |
March
2017 |
|
|
Inside
This Issue |
|
|
|
|
|
This e-newsletter is provided free to doctors through industry support from |
 |
| |
FROM
THE DESK OF THE EDITOR
As I was preparing this newsletter, an ORS colleague shared a paper out of Bascom Palmer describing three cases of bilateral vision loss caused by intravitreal injection of autologous stem cells. The journal is summarized in the abstract section below. Please take a look if you haven’t already read the paper. The story was picked up by many national news media outlets detailing a shady practice out to make a quick buck. This unfortunately occurred at the expense of a few venerable AMD patients desperate to improve their vision. While retinal stem cell therapy appears to be promising, there is still a long way to go. This paper highlights the importance of regulated, structured clinical trials
On a different note, I would like to acknowledge Dr. Steve Ferrucci for his time and effort serving as editor of this newsletter. While most ORS positions transition after a two year course, Steve has graciously held this position for almost 10 years. Now, as you’ll see below, he has been elected to ORS president. So it is time to pass the torch. Though I doubt I can last as long as he did in this role!
Anna Bedwell, OD, FAAO
Editor-in-Chief
PRESIDENT'S MESSAGE
As my two-year cycle of being the President of the ORS comes to a close,
For the past almost 10 years, I have been editor of the ORS Newsletter as well as Secretary at one point (which I was bad at) and Vice President most recently. Now, I am assuming the role of President of the ORS for the next two years. Honestly, I am very excited to take over this new role, as well as a little nervous. Excited as I love this group, and I hope to move it forward in new and exciting ways. Nervous, because I have some big shoes to fill of those that have come before me, such as Bill Jones, Jerry Sherman, Larry Alexander (miss you my friend) , Joe Pizzimenti and most recently Brad Sutton. All did a great job during their term, and I am just hopeful that I can continue the stellar track record.
Fortunately, I am not alone, and have a great supporting cast, with Jeff Austin as Vice President, Chris Suhr as treasurer for a second time (thanks Chris) and Julie Torbit as Secretary. I am still in the process of getting the rest of the committees set, but should have it solidified soon, and have an absolute fantastic group in mind. Anna Bedwell will be taking over my newsletter duties, and I am confident she will improve on the framework that I have supplied.
So, thank you to all my fellow ORS members who are allowing me to serve as your president. I will do my utmost not to disappoint!
Sincerely,
Steven Ferrucci, OD, FAAO
ORS President

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.

CLINICAL
PEARLS
Utilizing Fundus Autofluorescence in Clinical Practice
By Anna Bedwell, OD, FAAO
ORS Fellow
Fundus autofluorescence (FAF) has been around for decades but has more recently
reemerged. The technology is available with some retinal cameras and cSLO including the Heidelberg Spectralis. FAF assesses the health of the RPE by measuring the distribution of lipofuscin, a byproduct of photoreceptor outer segment turnover. With high levels of lipofuscin you will see hyperfluorescence indicating that the RPE is sick/stressed. Conversely, hypofluorescence indicates an absence of liposfuscin or atrophic RPE. FAF can reveal damage well before retinal changes are clinically visible. FAF is useful for many conditions including:
- AMD (particularly geographic atrophy)
- Central serous retinopathy
- Plaquenil toxicity
- Choroidal lesions (ie nevi vs melanomas)
- Glaucoma
- ONH drusen
- Macular and retinal dystrophies
For any outer retinal and choroidal disease this is a great tool to have in your arsenal!
|

JOURNAL
ABSTRACTS
Optical Coherence Tomography Angiography Versus Fluorescein Angiography In The Diagnosis Of Ischaemic Diabetic Maculopathy
OCT angiography is proving to be a very useful tool in the diagnosis and management of various retinal vascular diseases. While intravenous fluorescein angiography has been utilized in the management of these conditions for many years, OCTA holds the distinct advantage of being completely non-invasive.
This study compared the ability of these two similar yet distinctively separate techniques to detect and quantify ischemic diabetic maculopathy. Ischemic diabetic maculopathy is a well-known cause of vision loss in patients with diabetic retinopathy, and is particularly problematic due to the current lack of effective treatment options. In these patients, decreased foveal vascularization leads to capillary damage and a subsequent enlargement of the foveal avascular zone.
This study utilized both intravenous fluorescein angiography (IVFA) and OCT angiography (OCTA) to examine 31 eyes of 20 patients with ischemic diabetic maculopathy and 27 eyes of 17 control subjects. The foveal avascular zone was graded according to ETDRS criteria, and the researchers found that OCTA was equally as effective as the gold standard IVFA in imaging and evaluating ischemic diabetic maculopathy, especially when used to image the deep vascular plexus. It had the distinct advantage of not having its image obscured by dye leaking from the vasculature, and of course it is non-invasive, which is also a substantial advantage. The authors conclude that OCTA appears to be a highly effective and safe alternative to IVFA for evaluating diabetic macular ischemia.
Cennamo G, Romano MR, Nicoletti G et al. Acta Ophthalmol. 2017: 95: e36-e42
Optical Coherence Tomography Angiography In Retinal Vein Occlusion Treated With Dexamethasone Implant: A New Test For Follow-up Evaluation
OCT angiography, frequently referred to as OCTA, has become a valuable tool in the diagnosis and management of retinal vascular disease. This study examined the use of OCTA in monitoring changes in patients with retinal venous occlusions after the injection of a dexamethasone implant. A retrospective chart review was undertaken for seven eyes of seven patients who had their vein occlusions treated in this manner. Three patients had CRVO, while four had BRVO, all with substantial macular edema. Several factors were evaluated, with a comparison made between pre-implant and post-implant values. These included BCVA, central macular thickness (CMA), fluorescein angiography, and OCTA with an Optovue OCT. As expected, BCVA significantly improved (20/100 to 20/50 on average), and CMA significantly decreased (657 microns to 324 microns on average). Of note, changes in vascular density and capillary dropout could be appreciated utilizing OCTA imaging. With the use of the instrument’s proprietary analysis software, the changes could be quantified and followed over time, thus allowing for both qualitative and quantitative evaluation. The authors conclude that OCTA has promise as a non-invasive tool to follow retinal vascular changes in eyes with venous occlusions that have undergone intravitreal dexamethasone implants.
Glacet-Bernard A, Sellam A, Coscas F et al. Eur J Ophthalmol 2016; 26 (5): 460-468
Vision Loss After Intravitreal Injection Of Autologous “Stem Cells” For AMD
Stem cell therapy is an area of great promise in the treatment of many conditions and disorders, including macular degeneration. However, these potential therapies are very much still in the experimental scientific stages. Worldwide, there are 13 registered clinical trials on ClinicalTrials.gov that deal with intravitreal injections of various stem cells for the treatment of macular degeneration. Four of those trials are in the United States, only three of which are active and recruiting.
This article highlights devastating consequences that occurred in six eyes of three patients who received intravitreal injection of adipose tissue derived stem cells at a South Florida stem cell therapy clinic. This clinic was the site of the inactive clinical trial referred to above. Each of the three patients were elderly females with either geographic dry AMD or inactive neovascular AMD. Visual acuity in the six eyes prior to injection ranged from 20/30 to 20/200. Each patient paid $5,000.00 to have adipose derived stem cells harvested from their periumbilical region, processed, then injected bilaterally in to their vitreous on the same day. While the consent form signed by each participant indicated the possibility of blindness, it did not discuss participation in a clinical trial of any kind. Two of the three patients, however, had seen the site listed on ClincalTrials.gov before reporting to the clinic.
Within one week of treatment, all three patients began experiencing severe vision loss in both eyes and varying degrees of pain. All three reported to ophthalmologists for evaluation. Upon examination, a wide range of serious ocular complications were discovered. These complications included in various combinations lens dislocation, severe elevation of intraocular pressure, vitreous hemorrhage, retinal hemorrhaging, and retinal detachment. All patients experienced retinal detachment with proliferative vitreoretinopathy in at least one eye, as well as weakened lens zonules. These findings consistent across all three patients led the authors to speculate that the adipose stem cells may have transformed in to myofibroblasts. Tragically, in spite of extensive intervention, all six eyes ultimately lost vision. Final visual function was no light perception in two eyes, light perception on one eye, hand motion in two eyes, and 20/200 in one eye. This extremely unfortunate series of events reinforces the fact that desperate patients will often seek out and participate in unproven, untested, and unregulated procedures. While tight regulation of clinical trials can be frustrating and can potentially slow progress toward life changing therapies, it remains absolutely vital in helping to prevent catastrophes such as the one described here.
Kuriyan AE, Albini TA, Townsend JH, et al. N Engld J Med 376;11 NEJM.ORG
Neurological Complications of Acute Multifocal Placoid Pigment Epitheliopathy
AMPPE is a retinal inflammatory disorder characterized by the development of deep retinal placoid lesions. It tends to have a self-limiting course though does carry low risk of neurological complication.
This is a short case series highlighting 5 patients over a 5 year span with neurological complications related to AMPPE. The authors’ review of literature found only 47 other published patients with neurological involvement associated with AMPPE. There was a variety of neurological involvement reported including isolated headache, stroke/TIA, seizures, venous sinus thrombosis, optic neuritis, sensorineural hearing loss and peripheral vestibular disorder. Of the 52 cases reviewed, 31 (60%) had TIAs or strokes. These complications are presumed to be due to cerebral vasculitis and has been confirmed pathologically in three cases. Generally, the retinal disease preceded the headache or focal neurological deficits with over half of patients noticing neurological symptoms <4 weeks after ocular symptoms. Unfortunately, these patients have a poor prognosis as only 25/40 (62%) made a full recovery. The remaining cases had residual neurological disability and 5/40 (13%) died.
The authors recommended that patients presenting with AMPPE and neurological symptoms, including headache, should be worked up with MRI of the brain and CSF examination. Those with neurological symptoms of AMPEE should receive IV methylprednisone followed by a taper of oral steroids for at least 3 months.
Brownlee WJ, Anderson NE, Sims J, et al. Journal of Clinical Neuroscience. Sept 2016. 31:76–80.
Optical Coherence Tomography Angiography of Chorioretinal Diseases
OCTA allows for depth-resolved, three-dimensional images of the chorioretinal vasculature in a fast, noninvasive manner. The limitations include a restricted field of view, motion artifacts and the lack of leakage visualization and dye transit over time.
Since fluorescein angiography (FA) only allows assessment of the superficial retinal plexus, OCTA offers a better understanding of the diseases affecting deeper vascular segments of the retina, such as in type II macular telangiectasia and paracentral acute middle maculopathy. In diabetic retinopathy, OCTA can map out the size and shape of the foveal avascular zone and areas of poor retinal capillary perfusion. OCTA has the added value of determining the exact location of microaneursyms whether in the superficial or deep vascular plexus. OCTA also holds the advantage over FA in the ability of performing serial examinations, particularly with macular neovascularization.
Novais EA, Roisman L, de Oliveira PR, et al. Ophthalmic Surg Lasers Imaging Retina. Sept 2016. 47(9):848-861.
Intravitreal Bevacizumab for Proliferative Diabetic Retinopathy Results from the Pan-American Collaborative Retina Study Group (PACORES) at 24 Months of Follow-up
Panretinal photocoagulation has been the gold standard treatment for retinal neovascularization in PDR. However, PRP carries the side effects of peripheral field loss and macular edema and often requires more than one treatment to be effective. This has created a need for finding new treatment for PDR like anti-VEGF injections.
This was a retrospective, non-controlled study assessing the use of intravitreal bevacizumab on retinal neovascularization in PDR. The study evaluated 97 eyes at 24 month follow up with PDR related retinal neovascularization that had received at least one intravitreal bevacizumab injection ± PRP.
On average, there were 4 ± 2.5 injections per eye. Of the 60 eyes that had been treated > 6 months prior with PRP, 41 (68.3%) had good response to intravitreal bevacizumab alone.
The authors found that PDR was controlled or regressed with intravitreal bevacizumab in 42.1% (8/19) of treatment-naïve eyes. These eyes did not require additional laser or vitrectomy. They found intravitreal bevacizumab to be a promising supplement treatment to PRP for PDR.
Arevalo JF, Lasave AF, Wu L, et al. Retina. Feb 2017. 37:334–343.
Postoperative Hemorrhagic Occlusive Retinal Vasculitis
(Expanding the Clinical Spectrum and Possible Association with Vancomycin)
The intention of this study was to describe a syndrome of hemorrhagic occlusive retinal vasculitis (HORV) that presented following cataract surgery without apparent complication.
This was a retrospective case study comprised of 7 eyes of 4 patients collected from retinal specialists and combined with previously published case studies of an additional 4 eyes. All the eyes were similar in that they recently underwent uncomplicated cataract surgery including prophylactic intracameral vancomycin 1.0 mg/0.1 ml and experienced subacute (1-14 days) peripheral or central vision loss that was painless in nature. All eyes were remarkable for mild to moderate anterior and/or intermediate uveitis and hemorrhagic occlusive retinal vasculitis. HORV presents as diffuse occlusive retinal vasculitis with predilection for venules, extensive intraretinal hemes in ischemic regions and negative ocular and systemic work-up. All cataract surgeons were experienced and no similar findings were documented with other surgeries performed by the same doctor on same day. Fundus photography, fluorescein angiography, and SD-OCT imaging, along with extensive laboratory testing results are available to some extent for every presented case.
The authors concluded that while postoperative HORV is extremely uncommon, the condition is visually devastating and may represent a delayed immune reaction similar to vancomycin-induced leukocytoclastic vasculitis. Acute treatment with high-dose corticosteroids, antiviral medications and/or early vitrectomy was unsuccessful in encouraging visual recovery in the cases evaluated in this series. Clinical recommendations regarding the syndrome include avoiding routine prophylactic intracameral vancomycin use, allow 2-3 weeks to pass before operating on the fellow eye, and early anti-VEGF, panretinal photocoagulation to encouraged visual recovery and reduce the risk of neovascular glaucoma.
Andre J. Witkin, MD., Anjali R. Shah, MD., et al. Opthalmology. 2015 Jul;122(7):1438-51.
Atypical Presentation of Chorioretinal Folds-Related Maculopathy
Choroiretinal folds are undulation of the inner choroid, Bruch’s membrane and the retinal pigment epithelium that then indirectly impact overlying neurosensory retinal layers. Chorioretinal folds present a possible risk factor to develop retinal pigment epithelium atrophy and irreversible lesion to Bruch’s membrane, leading to an angiographic appearance that compare to angioid streaks. The causes of folds include hypotony, hyperopia, thyroid eye disease, uveal effusion syndrome, posterior scleritis, tumors, scleral buckling surgery, and age-related macular degeneration. Three specific stages of choroidal folds-related maculopathy have been identified, with the third potentially including choroidal neovascularization. The six patients analyzed presented with either occult form (type I) choroidal neovascularization, predominantly classical (type II) choroidal neovascularization, chronic central serous chorioretinopathy-like maculopathy with apparent telangiectatic retinal capillaries or simply chronic central serous chorioretinopathy-like maculopathy in one or both eyes.
This study presents retrospectively analyzed clinical data of six nonconsecutive patients diagnosed with chorioretinal folds-related maculopathy with atypical presentation and an idiopathic etiology. Testing included systemic workup, measurement of best-corrected visual acuity, fundus biomicroscopy, fluorescein angiography, indocyanine green angiography, and spectral domain optical coherence tomography (SD-OCT). BCVA ranged from 20/200 to 20/80 at initial presentation. Four of six patients received intravitreal antivascular endothelial growth factor therapy, one of which included photodynamic therapy. A fifth received exclusively photodynamic therapy while a sixth received intravitreal injections of sustained-released dexamethazone implant.
The authors concluded that choroidal vessel dilation and hypermeability might be involved in atypical presentations of chorioretinal fold-related maculopathy. Both the apparent undulating folds but also sub-/intraretinal fluid accumulation characterize this condition. These findings can result in focal RPE alterations, making the eye susceptible to progression of choroidal neovascularization or chronic central serous chorioretinopathy-like maculopathy, the final of which may or may not be accompanied by telangiectatic retinal capillaries. Anti-VEGF and photodynamic therapy were shown to be successful in managing choroidal neovascularization in chorioretinal folds-related maculopathy, but had limited efficacy on chronic central cerous chorioretinopathy-like maculopthy with minimal acuity improvement. In this case, intravitreal injection of a sustained-released dexamethazone implant provided much more successful treatment results.
Federico Corvi, Vittorio Capuano, et al. Optom Vis Sci. 2016 Oct;93(10):1304-14.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
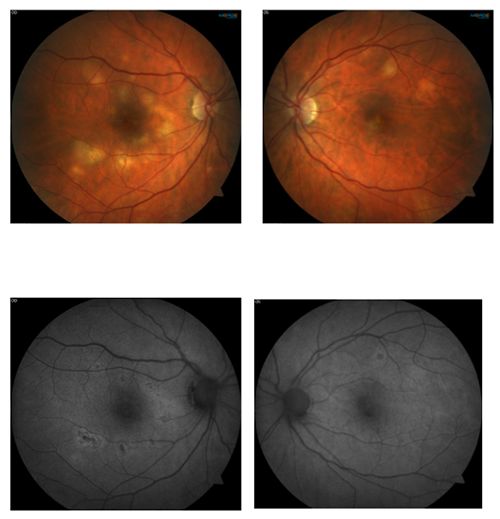
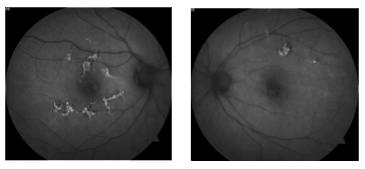
This 53 year old female patient presented symptomatic for visual disturbance and scotomas in her vision starting 3 days prior. She was ultimately diagnosed with acute multifocal placoid pigment epitheliopathy (AMPPE).
AMPPE, falling into the group of white dot syndromes, is characterized by outer retinal inflammation. It typically presents with bilateral yellow or creamy placoid lesions in the deep outer retina. AMPPE is more common in young adults of both sexes. Patients will sometimes report a viral prodome.
AMPPE typically runs its course over a few weeks. Oral steroids can help to hasten the resolution. The initially creamy lesions will fade into pigmented scarring. Prognosis is generally good for full vision recovery particularly if lesions spare the fovea. Depending on the location of the lesions persistent scotomas may be noted.
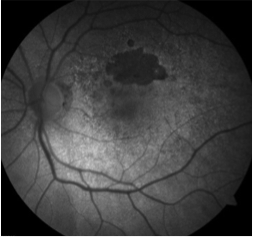
Fundus autofluorescence can be of particular use in differentiating AMPPE from other white dot syndromes. It will show hypoautofluorescence of the lesions in the acute phase as shown in the above FAF images. This is presumably from a masking effect by swollen photoreceptors. A few weeks in this changes to hyperautofluorescence as there is increased lipofuscin from the loss of photoreceptors. The FAF images below show the same patient 3 weeks after symptoms developed and after just finishing treatment with oral steroids. The now pigmented lesions prominently hyperautofluoresce on FAF.
Anna Bedwell, OD, FAAO
ORS Fellow
|

IN THE
NEWS
 |
Lucentis (ranibizumab, Genentech) received FDA approval for the treatment of patients with myopic choroidal neovascularization
Lucentis is now the first anti-vascular endothelial growth factor (VEGF) therapy approved by the FDA to treat myopic CNV in the US. This marks the fifth FDA-approved indication for Lucentis since its start in 2006. A phase 3 study showed better visual acuity gains in myopic CNV over verteporfin photodynamic therapy.
|
 |
Roche Acquires ForSight Vision4
Roche has acquired ForSight Vision4 and in turn its port delivery system (PDS) with hopes to incorporate this new technology to lower the treatment burden associated with intravitreal injections in AMD. The implant is placed through a scleral incision in a single surgical procedure. Physicians can then refill the implant using a proprietary needle every 4-6 months.
A phase 2 study is currently underway using ranibizumab PDS in exudative AMD ("LADDER" Study, NCT02510794, sponsored by Genentech). Data is expected out in late 2017.
|
 |
Recall: Vancomycin hydrochloride injection
Hospira, Inc. is voluntarily recalling 1 lot of vancomycin hydrochloride injection. The lot was sold from August 2016 through September 2016 in the United States USP (NDC: 0409-6510-01, Lot 591053A, Expiry Date NOV 2017). This is because of a confirmed customer report showing particulate matter inside a vial. Hospira has not received reports of any adverse events associated with this product.
|
 |
Combination Therapy Between Anti-VEGF and Topical timolol-dorzolamide Could Better Treat Macular Edema in Retinal Vein Occlusion
Though study results are still pending, David S. Ehmann, MD presented data to support the use of topical timolol-dorzolamide in conjunction with anti-VEGF therapy to treat macular edema secondary to retinal vein occlusion. The group has found improvement in vision and a significant reduction in central retinal thickness in patients with historically incomplete response to anti-VEGF therapy alone. This is a small study of only 10 patients but could lead to a larger study.
Ehmann D. Topical timolol-dorzolamide in combination with intravitreal Anti-VEGF for retinal vein occlusion macular edema. Presented at: Wills Eye Conference; March 9-11, 2017; Philadelphia.
|
 |
FDA Clears New Drug for Clinical Trial in Treatment of Ocular Melanoma
Aura biosciences has been cleared by the FDA to start its first human clinical trial for its AU-011 light activated drug. AU-011, administered through intravitreal injection, consists of viral nanoparticle conjugates that selectively bind to cancer cells sparing the surrounding healthy tissue. The drug is activated via an ophthalmic laser.
The FDA has approved orphan drug designation for AU-011 to treat ocular melanoma. Phase 1b clinical trial is in enrollment to evaluate two dose levels of AU-011 in the treatment of small-to-medium primary ocular melanoma.
|

MEET
THE FELLOWS Nick Fogt graduated from the Ohio State University College of Optometry in 1992 while also completing an M.S. degree (Physiological Optics) in the College of Optometry’s combined OD/MS program. Following graduation, Dr. Fogt completed a residency in hospital-based optometry at the Cleveland Veterans Administration Medical Center. He then returned to Ohio State, completing a PhD (Physiological Optics) in 1996. Dr. Fogt then took a position at the OSU College of Optometry as an Assistant Professor of Optometry and Physiological Optics. He is now a Professor at the OSU College of Optometry, where he continues to teach courses in posterior segment disease and systemic disease. In his free time, Dr. Fogt enjoys spending time with his two entertaining kids by teaching them about the retina and coaching their various sporting teams. Nick is a big OSU Buckeye sports fan and tries to cheer loudly for his team whenever possible.
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
SPONSOR NEWS


Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Jared Staats, OD
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|