| |
Volume 17, Number 3 |
September 2021 |
|
FROM
THE DESK OF THE EDITOR
One of my interests in the past few years has become inherited retinal disease (IRD). Go figure, I’m a member of a retina society and write the group’s newsletter. So, I think it is a given that I have an interest in the posterior segment. But what has really perked my interest in IRDs is the fact that IRD genetic testing has recently become readily available. There are two programs currently offering no-charge test kits. Genetic testing for an IRD used to be a very costly endeavor, often deterring patients. Now, with the availability to offer this at no cost, almost all of my patients have taken advantage.
When I first started offering this to patients with suspected IRD, I mistakenly thought it would be clear-cut. That I would get results back and give them firm answers. Well, it turns out after the first 10-page test report came back, I knew had a lot to learn and review. What have I learned? Well, sometimes the genetic test comes back with a clear answer that matches exactly what you expect clinically. And sometimes it doesn’t match at all. That could mean the particular mutation is on a gene that has yet to be mapped. So maybe years from now, we can retest and get an answer.
For some patients, the results turn up a bunch of markers with “uncertain significance,” a confusing alphabet soup. Maybe a patient has a combination of mutations that resulted in bad luck for the retina. I try to counsel patients accordingly. For most patients, the information isn’t life- or vision-changing. But when we can provide a proper diagnosis, it’s appreciated. And maybe someday soon with promising gene therapy trials, we’ll be able to offer them a treatment. Fortunately, with these programs comes an option to offer a visit with a genetic counselor. So if these programs are of interest to you, I recommend checking them out: https://www.invitae.com/en/idyourird/ and https://blueprintgenetics.com/my-retina-tracker-program/. Do your homework and then be able to provide a wonderful resource for patients. And if you’ve read this, I’ve given you a leg up on the image quiz below!All the best! Cheers!
Anna Bedwell, OD, FAAO, FORS
Editor-in-Chief
PRESIDENT'S MESSAGE
I will keep this issue’s “President Message” to a few bullets so that you can spend more time with the content of our newsletter that is put together by the hard work of Dr. Bedwell.
• We are finalizing the details of the Retina Update. For now, mark your calendars for December 11th and 12th, for a live meeting with a virtual simulcast at Hampton Beach, Calif. Stay tuned for more information.
• Thank you to the Fellows that submitted lectures; we will have a great meeting.
• I am proud to announce that the ORS Resident Case Report was published in the June issue of RVOP. Thanks to Dr. Rodman and those of you who helped raise scholarship funds, and recruited and encouraged the residents to submit papers, as well as the judges: https://www.reviewofoptometry.com/news/article/ors-resident-case-report-contest-winners-announced
• We are still looking for qualified candidates for Fellowship. Would you please send an email if you or someone you know is interested?
• Thanks to the efforts of Drs. Rodman and Austin, paying our dues has become much more manageable. If you have not yet paid your dues, please log in to the ORS website where you can pay by Paypal or CC: https://www.optometricretinasociety.org/membership-dues/
• If you have an interesting retinal case to share, please consider contacting Dr. Bedwell. She is always looking for great cases to share with our readers.
Be safe, keep in touch, and I hope to see many of you in December at the ORS Retina Update.
Mohammad Rafieetary, OD, FAAO, FORS
President, Optometric Retina Society

YOU
MAKE THE DIAGNOSIS
Answer appears later in newsletter.

Image Gallery
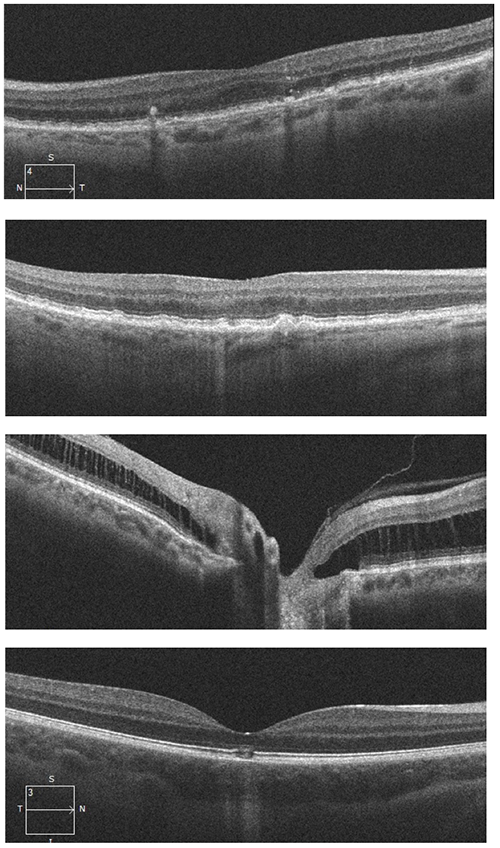
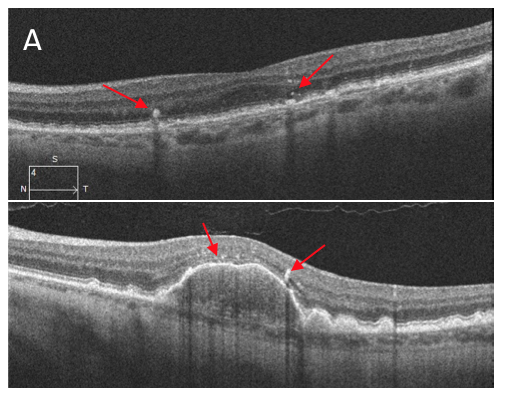
Which of the following OCT images represents hyperreflective foci?
Answer appears later in the newsletter.
|

JOURNAL
ABSTRACTS
Incidence and Risk Factors for Macular Edema after Primary Rhegmatogenous Retinal Detachment Surgery: A Prospective Single-Centre Study
Cystoid macular edema (CME) is a known complication of retinal detachment surgery. The incidence varies widely in the literature, with many studies from a pre-OCT era. This prospective study monitored rhegamotogenous retinal detachment (RRD) patients to determine postoperative CME rates as well as risk factors.
The study included patients with primary RRD repair (either scleral buckle, vitrectomy or combined) and excluded those with prior CME or epiretinal membrane (ERM). Of the initial 150 patients seen over a course of 18 months, 107 met the criteria and had retinal attachment at follow up. Most of the RRD repairs were scleral buckle 54.2%, follow by vitrectomy 25.2% and combination 20.6%. About half of those RRDs were macula-on and the rest macula-off. Participants were examined and had SD OCT at 3 weeks and 6 weeks postop. The frequency of CME was 18.7%, neurosensory detachment 31.8% and ERM 32.7%. CME developed in 14 eyes at 3 weeks, and 6 additional eyes at 6 weeks post op. In most of these cases, CME resorbed without treatment, only 4 cases necessitated treatment. By far, the risk of CME was higher in those that developed ERM at 42.9% vs. 6.9% (p<0.001). CME developed more often in those with a macula-off RRD (26.5% vs. 11.1%, p=0.044).At an incidence of 18.7%, this study shows that CME is a common postoperative complication of surgical RRD repair. As the authors note, SD-OCT allows for enhanced visualization for subtle cases of CME, giving an advantage over prior studies that relied on clinical exam and/or TD-OCT. Not surprisingly, ERM which can in itself cause CME due to tractional effects, is a major risk factor postoperatively. Fortunately, as this paper demonstrates many cases of CME will resorb without intervention.
Gebler M, Pfeiffer S, Callizo J, et al. Incidence and risk factors for macular oedema after primary rhegmatogenous retinal detachment surgery: a prospective single-centre study. Acta Ophthalmol. 2021; Jun 16. [Epub ahead of print].
Diagnostic Accuracy of Optical Coherence Tomography Angiography in the Detection of Neovasculature in Age-Related Macular Degeneration: A Meta-analysis
The noninvasive nature of OCT angiography (OCTA) makes it a desirable choice to monitor for choroidal neovascular membrane development in AMD. But how does the technology fair in comparison to gold standard fluorescein angiography? This meta-analysis started with a literature search focused on studies that examined patients with suspected or confirmed exudative AMD, included OCTA and FA, and relayed efficacy markers. Bias risk was assessed via the QUADAS-2 tool and Review Manager software. The meta-analysis included 553 eyes across seven diagnostic studies. The total sensitivity was 85.9%, and specificity was 89%. The total positive and negative likelihood ratios were 8.36 and 0.15, respectively. The limitations of OCTA were false negatives due to artifacts. Several of the studies reported false positives, including those caused by quiescent neovessels. Even though OCTA was quite sensitive and specific in detection of CNVM, the authors noted that the evidence did not show superiority over FA. That said, OCTA still holds promise as a faster, noninvasive technology that could in turn reduced the number of FAs performed.
Maesa JM, Baños-Álvarez E, Rosario-Lozano M-P, et al. Diagnostic accuracy of optical coherence tomography angiography in the detection of neovasculature in age-related macular degeneration: A meta-analysis. Acta Ophthalmol 2021; Jul 26. [Epub ahead of print].
Transocular Sonography in Acute Arterial Occlusions of the Eye in Elderly Patients: Diagnostic Value of the Spot Sign
Central retinal artery occlusion (CRAO), branch retinal artery occlusion (BRAO), and anterior ischemic optic neuropathy (AION) are three of the most common causes of ischemic vision loss. Giant cell arteritis should always be considered in cases of AION, as prompt diagnosis and treatment are essential. Patients diagnosed with non-arteritic AION and those with retinal artery occlusions often share significant clinical characteristics, including a high or very high cardiovascular risk profile. CRAO and BRAO are often caused by an embolic artery occlusion, while non-arteritic AION are believed to be caused by a compromised oxygen supply to the optic nerve head. Transocular B-mode ultrasound allows for visualization of these emobli as a hyperechoic spot seen centrally in the optic nerve and labeled the “spot sign.” This spot sign is of clinical importance in individuals with CRAO due to its high negative predictive value of cranial GCA. A retrospective study of patients, all age ≥50, with documented CRAO, BRAO, or AION that were referred for vascular ultrasounds was performed. A diagnosis of cGCA was noted in 50% of the AION patients, 6.5% of patients with CRAO and none of the BRAO patients. Cardiovascular comorbidities were not found to have clinical significance between the CRAO, BRAO, and AION patients in this study. The spot sign was present in 39 patients (32%), none of which had AION. It was found in 32 of the 46 CRAO patients (69.6%) and in 7 of the 23 BRAO patients (30.4%). Four CRAO patients who were assessed on the same day as symptoms onset all had a positive spot sign. The presence of arterial occlusions with a positive spot sign is highly specific for an embolic CRAO diagnosis when located within the optic nerve. It also has a 100% negative predictive value for arteritic CRAO secondary to cGCA, but does not differentiate between an arterial or cardiac embolic source.
Czihal M, Lottspeich C, Köhler A, et al. Transocular sonography in acute arterial occlusions of the eye in elderly patients: Diagnostic value of the spot sign. PLoS One. 2021;16(2):e0247072.
The Association of Stroke With Central and Branch Retinal Arterial Occlusion
Central retinal artery occlusions are known to have a strong association with cerebrovascular accidents (CVA). The American Heart Association recommends a stroke workup for any RAO or amaurosis fugax patient, but surveys have shown that less than 40% of ophthalmologists promptly refer RAO patients for a stroke workup. The aim of this study was to determine the near-term risk of stroke after a RAO. The self-controlled case series (SCCS) included 16,193 RAO patients all over the age of 55, with the average age being 74.6. In total, 394 strokes occurred within the 6 months preceding and proceeding the RAO. The week prior to the RAO had the second highest recorded stroke rate, and the subsequent week had the highest stroke rate; the same was true when evaluating on a monthly basis.
The propensity score-matched cohort study included 18,213 RAO patients that were matched with 18,213 hip fracture patients. Hip fracture patients were used since both RAO patients and hip fracture patients are classically older. New strokes were seen in 1,807 of the RAO group, while only 606 strokes occurred in the hip fracture patients.Overall, this demonstrates that individuals presenting with a RAO have an increased stroke risk and should be emergently evaluated.
Scoles D, McGeehan B, VanderBeek BL. The association of stroke with central and branch retinal arterial occlusion. Eye (Lond). 2021 Apr 28. [Epub ahead of print].
Peripapillary Hyper-reflective Ovoid Mass-like Structure (PHOMS): An Optical Coherence Tomography Marker of Axoplasmic Stasis in the Optic Nerve Head
Peripapillary Ovoid Mass-like Structure (PHOMS, pronounced “fomms”) is an OCT finding best appreciated with enhanced depth imaging. PHOMS represent herniated optic nerve fibers bulging into the peripapillary region in discs with axoplasmic stasis. They wrap around the nerve in a centrifugal fashion, and the cross-sectional slices seen on OCT reveal a round, diffusely hyperreflective structure adjacent to the disc margin just above Bruch’s membrane. A good analogy is to think of a donut or inner tube-shaped structure wrapping around the optic disc. PHOMS deflect some of the overlying retinal layers up and over them in a “ski-slope” appearance. They appear different from optic disc drusen in that they are hyperreflective instead of optically hollow, and they are located outside of the optic disc margins. They appear different than blood vessels because they are located outside of the disc and do not have hyperreflective borders like blood vessels often do. PHOMS are only detectible with OCT and cannot be imaged with any other technology, and they have been seen to disappear when underlying disc edema resolves. They have been reported in many conditions that lead to axoplasmic stasis including papilledema, optic disc drusen, NAION, and tilted discs.
Fraser JA, Sibony PA, Petzold A, et al; ODDS Consortium. Peripapillary hyper-reflective ovoid mass-like structure (phoms): an optical coherence tomography marker of axoplasmic stasis in the optic nerve head. J Neuroophthalmol. 2021; Feb 19. [Epub ahead of print].
Acute Central Retinal Artery Occlusion Seen Within 24 Hours at a Tertiary Institution
Central retinal artery occlusion is a devastating ocular condition that often results in severe loss of vision. It is a form of ischemic stroke and has been found to be contiguously associated with neurological stoke in approximately 25% of cases. Treatment options are limited and have been notoriously infective historically. One treatment option that has shown some promise but remains controversial is intravenous thrombolysis within 4.5 hours of the onset of vision loss. This report examined CRAO patients reporting to Emory University’s emergency department and ophthalmology clinic between 2010 and 2020. Over the entire decade studied, only 181 patients met the criteria to be considered. Seven patients were excluded due to “medical complexity,” leaving 174 in the study. Twenty-one patients presented within 4.5 hours of noticing the onset of vison loss, 41 presented between 4.5 and 24 hours, and 112 presented after 24 hours. This distribution highlights just how uncommon it is for patients to present within the 4.5-hour window to be considered for thrombolysis therapy. At a prestigious medical center such as Emory, only 21 patients did so in an entire decade. Interestingly, only 3 of these 21 patients who presented within 4.5 hours received intravenous thrombolysis. The low number of interventions was the result of a combination of delayed diagnoses, contraindications to intravenous thrombolysis, and patient refusal. Also, four of the patients were excluded from possible thrombolysis because they noticed the vision loss upon awakening, so even though they arrived within 4.5 hours of noticing the vision loss, the true duration of the loss could not be determined. The only other advanced intervention attempted was hyperbaric oxygen therapy on two patients, both of whom presented over 24 hours from noticing vision loss.
Chan W, Flowers AM, Meyer BI, et al. Acute central retinal artery occlusion seen within 24 hours at a tertiary institution. J Stroke Cerebrovasc Dis. 2021; Sep;30(9):105988.
Safety and Efficacy of Intravitreal Risuteganib for Non-Exudative AMD: A Multicenter, Phase IIa, Randomized, Clinical Trial
Risuteganib, a peptide integrin regulator, is a drug showing the ability to protect the retinal pigment epithelium from oxidative stress, a key player in the development of age-related macular degeneration (AMD). This Phase IIa, prospective, double-masked study evaluated the efficacy of 1.0mg intravitreal risuteganib in patients with nonexudative AMD. Forty patients were randomized into either the treatment group (25 patients) or the sham group (15 patients). The primary goal was to determine the percentage of patients showing an 8-letter ETDRS improvement in best-corrected visual acuity (BCVA) in the two groups. All patients were examined every 4 weeks for 32 weeks. Participants received either 1.0mg/0.05mL risuteganib or sham injection at day 0 and week 16. In this study, 48% (12/25) of patients receiving two risuteganib injections gained 8 or more ETDRS letters at 28 weeks compared to 7.1% (1/14) of patients receiving a sham injection at 12 weeks. Twenty percent of those receiving two risuteganib injections gained more than 15 ETDRS letters at 28 weeks compared to 0% in the sham injection at 12 weeks. The mean BCVA of letters gained in the treatment group at 28 weeks was 12.8. The peak treatment effect occurred 12 weeks after a risuteganib injection. After 12 weeks, BCVA showed a mild decline until 16 weeks when a second injection could be administered. Repeated injections showed an additive effect to the prior dose. These results suggest risuteganib durability in dry AMD is every 12 weeks. Those who crossed over to receive one risuteganib injection showed similar effects. There were two patients in the treatment group who converted from dry AMD to wet AMD, compared to zero in the sham group. Given the small sample size, it was difficult to interpret if this conversion was drug related. This will be monitored more closely in future studies.
Boyer DS, Gonzalez VH, Kunimoto DY, et al. Safety and efficacy of intravitreal risuteganib for non-exudative amd: a multicenter, phase 2a, randomized, clinical trial. Ophthalmic Surg Lasers Imaging Retina. 2021; Jun;52(6):327-35.

|
ANSWER
TO "YOU MAKE THE DIAGNOSIS"
Sector Retinitis Pigmentosa
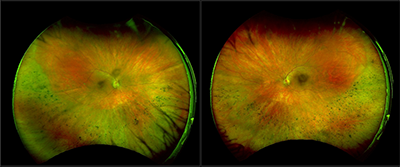
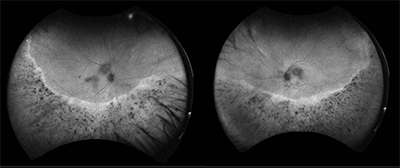
A forty-seven-year-old Caucasian male was referred for evaluation of inferior chorioretinal atrophy OU. His only visual complaint was poor night vision with difficulty seeing in low light conditions. His medical history was significant for hypertension and hyperlipidemia, which were controlled with medications. He reported no family history of ocular conditions. Upon evaluation, his best-corrected visual acuity was 20/20 in each eye. His intraocular pressure measured 18 mmHg in both eyes. Humphrey 30-2 visual field testing performed at his referring doctor’s office revealed superior defects in both eyes. All other entrance testing was normal. Both anterior segments were normal as well other than mild PSC cataracts in both eyes. Fundus evaluation revealed symmetrical, inferior chorioretinal atrophy with bone spicule pigmentation in both eyes. Fundus autofluorescence revealed patchy, inferior hypofluorescence with a hyperfluorescent border. Macular OCT testing performed at the referring doctor’s office was normal. Full-field ERG testing performed at the referring doctor’s office revealed depression OS>OD. Given the symmetrical fundus appearance in both eyes, the diagnostic testing results, and the presence of PSC cataracts OU, sector retinitis pigmentosa was suspected. Genetic testing confirmed this suspicion. The patient was heterozygous for autosomal recessive RP gene RBP3. In addition, he carried 9 mutations of uncertain significance, many of which were tied to RP. One of them, CDH 23, has been associated specifically with sector RP. The patient was counseled regarding the natural history of his condition, the lack of proven treatment options, his genetic test results, and the potential impact on his children.
Sector RP is an atypical form of RP, affecting only one or two quadrants. It is the result of mutations in the rhodopsin and cadherin 23 genes (RHO, USHIC, CDH23) and is usually bilateral and symmetrical. Unilateral cases have been reported. It is most often encountered in the inferior quadrants and is slowly progressive. The predilection for the inferior retina may be due to increased light exposure there compared to elsewhere in the retina. Like other forms of RP, it affects night vision and leads to visual field defects in the areas of the visual field that correspond to the involved sectors of the retina. Unlike other forms of RP, central vision is often spared or minimally affected. Various forms of multimodal imaging are helpful in making a diagnosis of sector RP, but fundus autofluorescence is particularly useful.
Brad Sutton, OD, FAAO, FORS
Clinical Professor, Indiana University School of Optometry
Service Chief, Indianapolis Eye Care Center
References:
1) Coussa RG, Basali D, Maeda A, et al. Sector retinitis pigmentosa: Report of ten cases and a review of the literature. Molecular Vision 2019; 25: 869-89.
2) Napier ML, Durga D, Wolsley CJ, et al. Mutational analysis of the rhodopsin gene in sector retinitis pigmentosa. Ophthalmic Genet 2015; 36: 239-43.
3) Daiger SP, Sullivan LS, Bowne SJ. Genes and mutations causing retinitis pigmentosa. Clin Genet 2013; 84: 132-41.
|

IN THE
NEWS
|
 FDA Grants Approval for AMD Biosimilar, Byooviz FDA Grants Approval for AMD Biosimilar, Byooviz
Byooviz, a biosimilar to Lucentis (ranibizumab) developed in a joint venture between Samsung Bioepis and Biogen, has been FDA approved under three uses: wet AMD, macular edema from retinal vein occlusion, and for myopic choroidal neovascularization. This marks the first ophthalmology biosimilar to be approved by the FDA. The hope is that Byooviz will offer a lower cost option to the currently approved anti-VEGF treatments, though the potential price has yet to be announced. To abide with a license agreement with Genentech, Byooviz will not be marketed until June 2022.
|
|
 Regeneron Ends Collaboration With Ocular Therapeutix Regeneron Ends Collaboration With Ocular Therapeutix
In a press release, Ocular Therapeutix announced that Regeneron ended the collaboration agreement across the two companies. The collaboration started in 2016 to develop a sustained-release anti-VEGF product utilizing Ocular Therapeutix’s sustained-release hydrogel technology. The effective termination date was August 5.
|
|
 FDA Grants Orphan Drug Designation for Aldeyra Therapeutic’s ADX-2191 FDA Grants Orphan Drug Designation for Aldeyra Therapeutic’s ADX-2191
In an August press release, Aldeyra Therapeutics announced that ADX-2191 was given orphan drug designation by the FDA for the treatment of retinitis pigmentosa (RP). ADX-2191 is an intravitreal injection of methotrexate that works to inhibit dihydrofolic reductase. Prior to this, ADX-2191 was granted orphan drug designation for treatment of primary vitreoretinal lymphoma and prevention of proliferative vitreoretinopathy. The orphan drug designation program helps development of treatment for rare diseases, such as RP, by providing sponsors with financial incentives. |
|
 Adverum Provides Update on ADVM-022 Development Program Adverum Provides Update on ADVM-022 Development Program
In a July press release, Adverum Biotechnologies generated an update on ADVM-022, a one-time intravitreal injection with an aflibercept coding sequence, based on a thorough review of its INFINITY (DME) and OPTIC (wet AMD) trials. This update came in response to a case reported in spring 2021 of Suspected Unexpected Serious Adverse Reaction (SUSAR) of hypotony in an eye treated with a single high dose of ADVM-022 for DME in the INFINITY trial. Adverum’s update notes a significant difference in the safety profile between the two studies, and between the low (2 x 10^11 vg/eye) and high (6 x 10^11 vg/eye) doses. On review of INFINITY, additional patients experienced serious adverse events related to hypotony occurring 16 to 36 weeks after treatment with the high dose. To date, no similar events have been observed in DME treated eyes with the low dose, or any wet AMD eyes with either the high or low dose. Development of ADVM-022 for DME has been halted due to dose-limiting toxicity not seen before in ocular gene therapy or anti-VEGF treatment. Adverum anticipates a Phase II clinical trial of ADVM-022 (2 x 10^11 vg/eye and lower) for wet AMD and to explore alternative prophylactic regimens.
|
| |
 Apellis Plans for New Drug Application for Geographic Atrophy Treatment Apellis Plans for New Drug Application for Geographic Atrophy Treatment
Apellis announced mixed results from Phase III trials in the treatment of geographic atrophy (GA) AMD with intravitreal pegcetacoplan, a targeted C3 therapy. In OAK, the primary endpoint was met as GA lesion growth was reduced by 22% and 16% in the monthly and every other month treatment groups, respectively. However, DERBY did not meet the primary endpoint, as GA lesion growth was only reduced by 12% and 11% in the monthly and every other month treatment groups, respectively. In the combined analysis of the two trials, those with extrafoveal lesions fared even better as treatment decreased GA lesion growth by 26% (p<0.0001) in the monthly group and by 23% (p=0.0002) in every other month group at 12 months. There were no safety concerns across both trials. Apellis plans to submit for new drug application to the FDA in early 2022. As it stands, there are no treatments approved for GA.
|
|
 MeiraGTx Announces Positive Data for AAV5-RPGR MeiraGTx Announces Positive Data for AAV5-RPGR
MeiraGTx presented data from its Phase I/II gene therapy study at the EURETINA 2021 meeting. MGT009 is an investigational study of a gene therapy, AAV5-RPGR, in the treatement of X-linked retinitis pigmentosa. In 10 adult subjects at 12 months post-treatment, AAV5-RPGR reversed the course of disease progression in comparison to the retinal function of those same subjects at 48 months pre-treatment. MeiraGTx and its partner, Janssen pharmaceuticals, are planning for the next step, the Phase III Lumeos clinical trial.
|

|
IMAGE QUIZ ANSWER
Image A (top) is the correct answer demonstrating hyperreflective foci (red arrows), which are dot-shaped lesions located at the apex of drusen on OCT. These foci often correspond clinically to focal hyperpigmentation and thus likely represent pigment granules. The foci originate in the outer retina and can migrate inwards over time. The presence of hyperreflective foci in dry AMD is associated with a five times higher risk of geographic atrophy within two years.
B: sub-RPE hyperreflective column C: peripapillary schesis
D: laser pointer maculopathy
|
WHY BECOME AN ORS FELLOW?
By Bill Denton, O.D., F.A.A.O.
Chair, Membership Committee
At some point in your career, you realize you just may be coasting. Your knowledge has been limited to the journals you receive and attempt to read, and the conferences that may not be as fulfilling as they once were. You simply need a challenge that will add an extra dimension to your professional learning.
Fellowship in the Optometric Retina Society (ORS) can provide several benefits in addition to the initial challenge of qualifying for this honor. Plenty of perks accompany your induction, but the coolest part is being associated with a body of knowledge and resources which can help you in many other ways. It is not uncommon to receive weekly thought-provoking emails about challenging cases and treatment dilemmas. Some fellows like to share their awesome cases they have diagnosed, while others post their cases with hopes that other Fellows will suggest an alternative differential diagnosis. At times it is like a round-table of brainstorming, but through the use of modern technology. Fellowship has little obligation with a huge opportunity for professional growth.
If you are up to the challenge of becoming a Fellow of the ORS, feel free to peruse the details and application at www.optometricretinasociety.org. Advice can be given to assist you in your quest. Feel free to contact us.
|
Editor
in Chief
Anna K. Bedwell, OD, FAAO
Co-Editor
Brad Sutton, OD, FAAO |
Journal
Reviewers
Jenna Wellen, OD
Brooke Tobias, OD
Senior Graphic Designer
Matt Egger
|
Review of Optometry® is published by the Review Group, a Division of Jobson Medical Information LLC (JMI), 11 Campus Boulevard, Newtown Square, PA 19073.
To subscribe to other JMI newsletters or to manage your subscription, click here.
To change your email address, reply to this email. Write "change of address" in the subject line. Make sure to provide us with your old and new address.
To ensure delivery, please be sure to add revoptom@lists.jobsonmail.com to your address book or safe senders list.
Click here if you do not want to receive future emails from Review of Optometry. |
|