Clinical Guide to Ophthalmic Drugs
—May 2011
Read PDF Edition
Welcome to the 2011 Clinical Guide to Ophthalmic Drugs!
This year, we are attempting to answer many of the questions we have received during our lectures over this past year. We have collected well over 100 questions, and we are sharing our responses to as many of them as space allows. We encourage you to read this question-and-answer dialogue, as it contains many clinically practical pearls that we trust you will value.
There have been two significant additions to the therapeutic landscape during the past year: Zirgan and generic latanoprost.
But there are also numerous "new and improved" remakes and enhanced formulations of medicines already in the marketplace:
- an increased concentration of gatifloxacin (Zymaxid, which is a 0.5% formulation)
- a decreased concentration of bimatoprost (Lumigan 0.01%)
- a decreased concentration of dexamethasone combined with tobramycin (TobraDex ST)
- another topical antihistamine for once-daily use (Lastacaft)
- a reformulation of moxifloxacin (Moxeza)
- a newer, lipid-based artificial tear (Systane Balance)
- the first once-daily topical NSAID, bromfenac (Bromday)
- loteprednol ophthalmic ointment (Lotemax ointment)
So, you can see the waters have been stirred! We will try to put these changes, and other relevant topics, into a clinically practical perspective for you. It must be absolutely stressed that everything written in this guide is explicitly aimed at enhancing the lives of the patients we all serve. We can never lose sight of why we exist and what our mission is.
With all best wishes to our esteemed colleagues,
Ron Melton, O.D. Randall Thomas, O.D., M.P.H.
Additional Publications
-
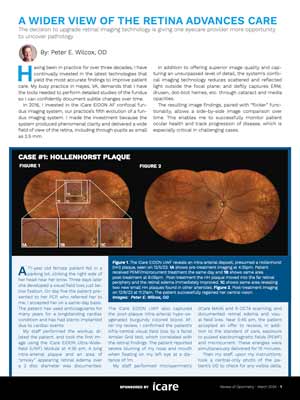
A Wider View Of The Retina Advances Care
The decision to upgrade retinal imaging technology is giving one eyecare provider more opportunity
to uncover pathology.Sponsored by iCare
It’s Time to Talk to Your Patients about Digital Eye Strain
New Developments in Glaucoma
Ophthalmic Product Guide - February 2024
Preservatives in Eye Care: Intrepid Eye Society Consensus Discussion
2024 Conference Planner