 |
|
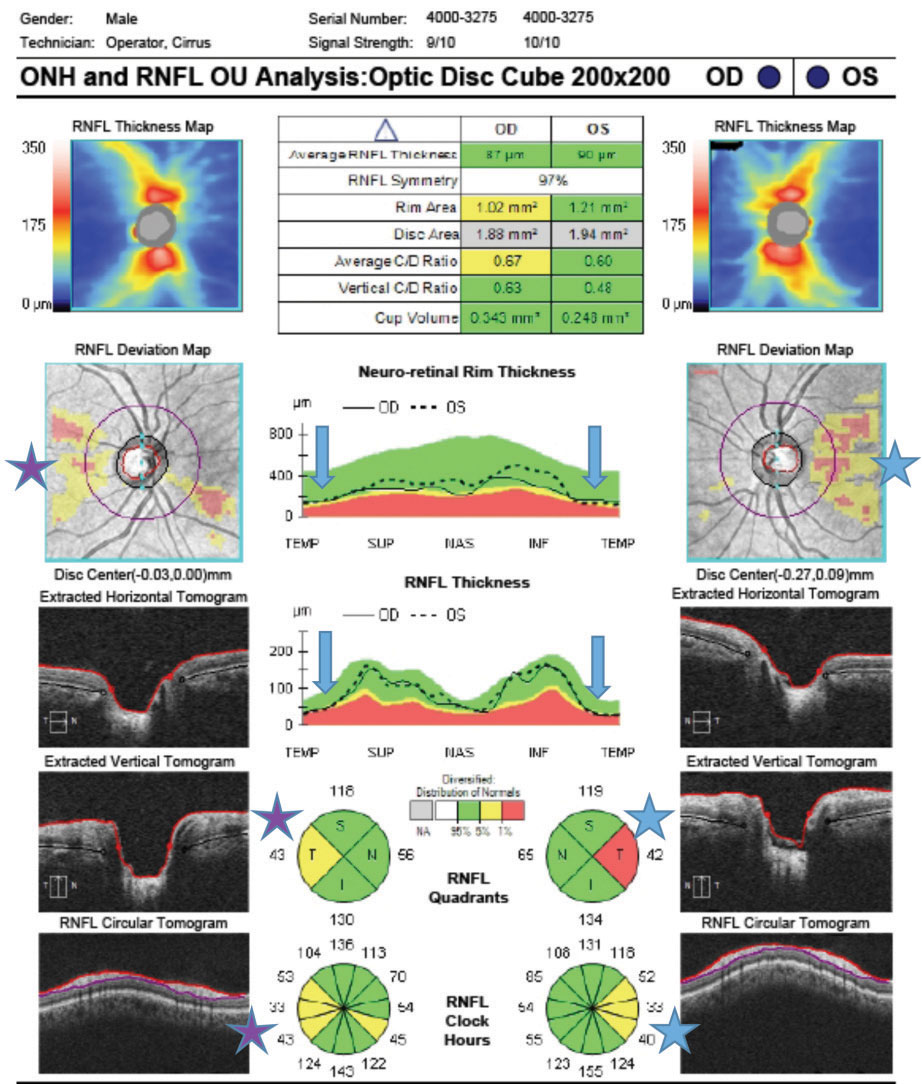
This study suggests that with further research, OCT biomarkers may be able to detect dementia in its early stage. Photo: Jarett Mazzarella, OD, and Justin Cole, OD. Click image to enlarge. |
With many ocular biomarkers having been proposed to detect early Alzheimer’s disease (AD) and mild cognitive impairment (MCI), many systematic reviews have been conducted. One new study takes a closer look at these recent reviews, specifically aiming to assess the diagnostic accuracy of each proposed biomarker for early dementia diagnosis.
In total, 14 systematic reviews were included. Three different biomarkers provided the most evidence in AD detection compared to the others, including OCT peripapillary retinal nerve fiber layer thickness, OCT-A foveal avascular zone and saccadic eye movements as well as antisaccade latency or error, which displayed the best accuracy.
With modest diagnostic performance for OCT and OCT-A, the authors highlight that these techniques may still provide use in dementia investigation. One possibility is to analyze changes in OCT parameters over time for the same individual, which might help aid in detection. Subsequently, they propose the implementation of longitudinal studies on AD and MCI that might result in better diagnostic accuracy.
The researchers do note that compared to other, more widely accepted biomarkers to assess dementia, such as cerebrospinal fluid or positron emission tomography, ocular biomarkers still hold the issue of needing to test for sensitivity and specificity. Comparing this information with diagnostic yield populations selected on a clinical diagnostic basis would help clarify whether the weaker diagnostic performance was in fact real or caused by loose clinical diagnostic criteria seen across the studies included.
Based on these three ocular biomarkers, the authors suggest, in a paper for JAMA Ophthalmology, that “future studies on eye imaging biomarkers in dementia should clearly report diagnostic criteria for dementia/MCI, whether the diagnosis is clinical or supported by biomarkers, the staging of the disease, longitudinal follow-up (when available) and post-mortem neuropathological confirmation.”1
That’s not all, though. An invited commentary on the study further elucidates the work’s key points into larger clinical translations. This consists of two main considerations.
First, that repeated screenings of patients using change measures as biomarkers might serve better than static measurements. For instance, retinal thinning, when measured longitudinally, may be a better indication than any one measurement in isolation. Additionally, the commenter points out that Alzheimer’s can be very difficult to parse out from other neurocognitive aging disorders and becomes even more complex when the standard criteria for diagnosis do not always highly correlate with clinical symptoms. The difficulty should be apparent, as ocular biomarkers most closely would detect early stages of Alzheimer’s, when treatment options are most effective.
This commentary’s main takeaway is that “the eye has long been feted as a potential biomarker for neurodegenerative diseases, including AD, for several reasons; namely, its ease of accessibility and the widespread availability of noninvasive imaging techniques, which also makes it a good candidate for population screening.”2
Another commentary in the same issue of JAMA Ophthalmology takes a different approach, though, focusing on apolipoprotein E (apoE), encoded by the APOE gene. The gene is known to be associated with Alzheimer’s, with high plasma apoE and a specific type of APOE allele associated with lower AD risk. In AMD, that specific APOE allele and higher plasma apoE are associated with lower AD risk and associate with AMD risk.
The second commentary highlights that the complex relationship between apoE levels, APOE variation, AD risk and AMD risk should be considered. To relate to the original study’s aim of providing clearer diagnostic biomarkers for the disease, this commentary’s authors believe that “this important work warrants attention and careful consideration for the apoE levels as a therapeutic target for AD.”3
What is clear throughout all three pieces is the attention to relating ocular biomarkers to clinical and real-world application, with each honing in on one aspect they believe to be important moving forward with further investigation between ocular connections to Alzheimer’s and other dementia types.
1. Costanzo E, Lengyel I, Parravano M, et al. Ocular biomarkers for Alzheimer disease dementia an umbrella review of systematic reviews and meta-analyses. JAMA Ophthalmol. November 17, 2022. [Epub ahead of print]. 2. Barret-Young, A. An eye on clinical translation—considerations for research into ocular biomarkers of Alzheimer disease and related dementias. JAMA Ophthalmol. November 17, 2022. [Epub ahead of print]. 3. Grunin M, Kinzy TG, Cooke Bailey JN. Note the nuance—examining the association of Alzheimer-protective APOE variation with risk for age-related macular degeneration. JAMA Ophthalmol. November 17, 2022. [Epub ahead of print]. |


