 |
|
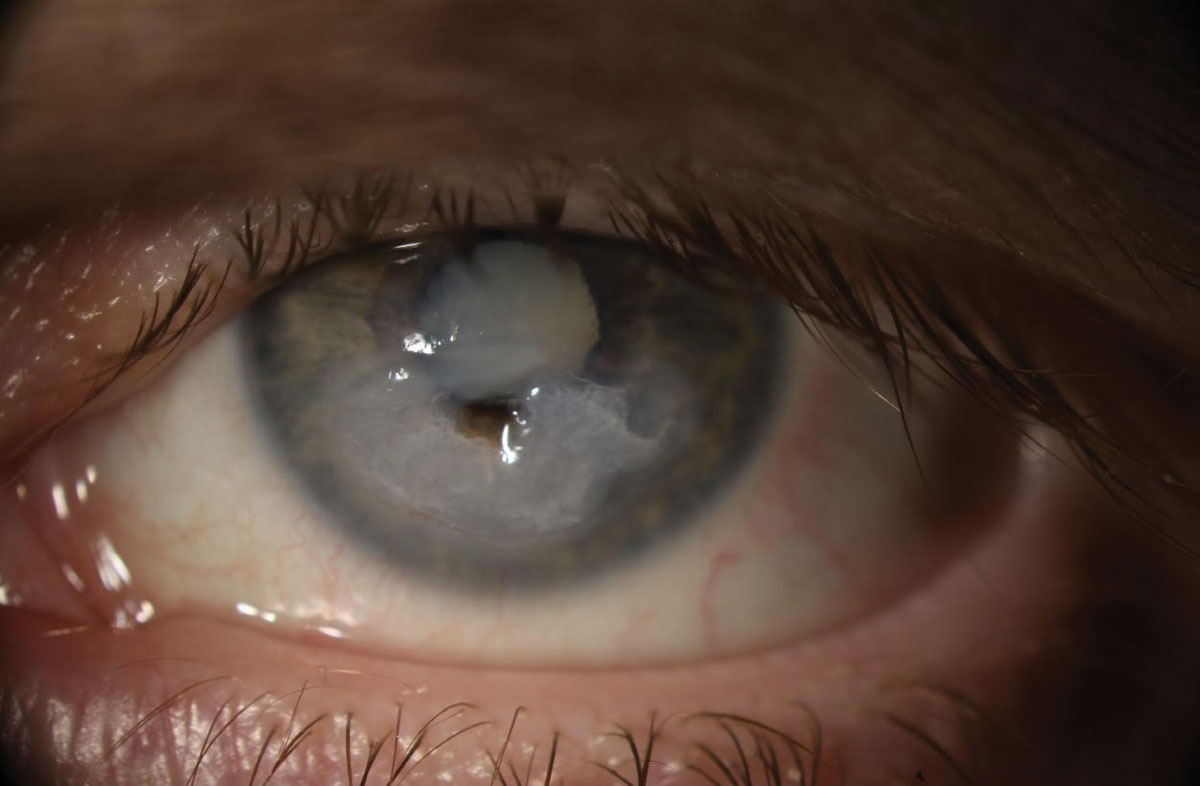
In stage I of neurotrophic keratopathy, rhNGF seemed to be well-tolerated. Photo: Megan Mannen, OD. Click image to enlarge. |
While recombinant human nerve growth factor (rhNGF) is FDA-approved for all stages of neurotrophic keratopathy, clinical trials to date have only included patients with stage II and III disease. A recent retrospective study, which sought to better understand the impact of this treatment among patients with stage I neurotrophic keratopathy, found that rhNGF (cenegermin) therapy was well-tolerated in this group.
The analysis included 17 patients with stage I neurotrophic keratopathy from three sites who were treated with rhNGF for eight weeks. Central corneal IVCM and image analysis was performed. In cases where IVCM was available before and after treatment (n=5), the researchers compared nerve density with age- and sex-matched healthy controls (n=13).
Prior to undergoing treatment, the baseline mean±SD corneal fluorescein staining score was 4.0±1.0, according to the study authors, who reported a significant improvement after therapy with rhNGF (1.06±0.77). The mean BCVA of patients at baseline was 20/40. This improved to 20/30 following the eight-week treatment protocol.
There was no vision reduction among patients using this topical agent. While 10 patients (59%) reported mild to moderate discomfort while on rhNGF, this did not lead to any interruption or discontinuation of treatment, the study authors noted.
Following eight weeks of rhNGF treatment, the researchers observed an increase in the mean total nerve density, main trunk nerve density and branch nerve density when compared with baseline. They calculated the average speed of nerve regeneration as 1.87mm/mm2 per month.
“This case series demonstrates that topical rhNGF treatment for neurotrophic keratopathy stage I increased corneal nerve density in a subset of our patients after rhNGF therapy, who initially presented with severe nerve loss,” the study authors wrote in their paper. “Moreover, we show that as a result of rhNGF treatment, BCVA and corneal fluorescein staining scores significantly improved. Furthermore, rhNGF was well-tolerated with minimal adverse events in these patients.”
Saricay LY, Bayraktutar BN, Lilley J, et al. Efficacy of recombinant human nerve growth factor in stage I neurotrophic keratopathy. Ophthalmology. August 13, 2022. [Epub ahead of print]. |


