 |
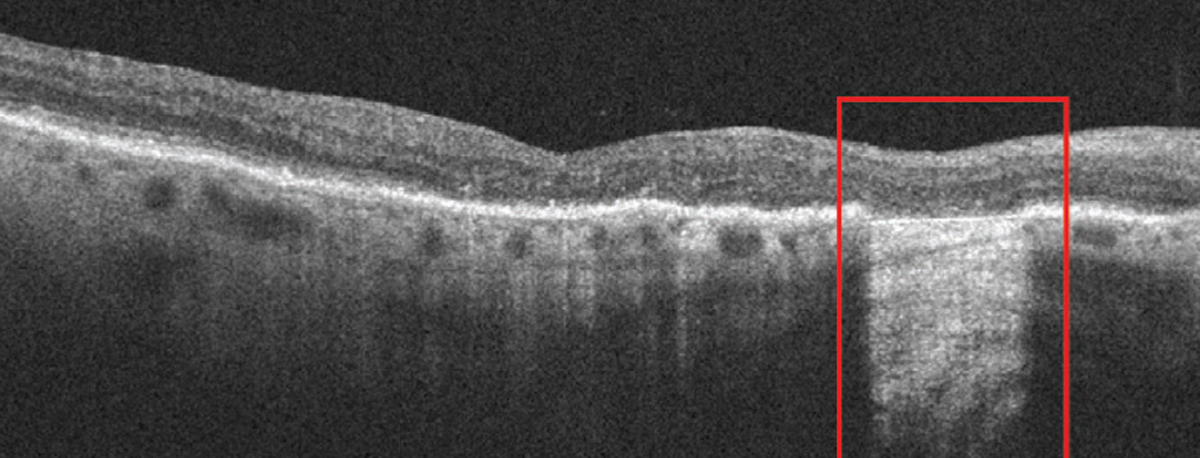
| Early cRORA lesions have a slower growth rate in comparison to atrophic lesions in advanced disease stages of GA. Photo: Anna Bedwell, OD. Click image to enlarge. |
Artificial intelligence (AI) is changing the way researchers investigate certain diseases and progressions, such as geographic atrophy (GA) secondary to dry age-related macular degeneration (AMD). In a recent study, researchers investigated the progression of early- and later-stage lesions using AI-based precision tools and found significantly slower disease progression in recently developed complete retinal pigment epithelial and outer retinal atrophy (cRORA) lesions measured on SD-OCT. According to the authors, this is the first study of its kind.
A total of 74 eyes of 49 patients with at least one cRORA lesion secondary to AMD were included and imaged by SD-OCT every six months over 18 months. Patients were divided between recently developed cRORA lesions and lesions with advanced disease status, and growth rates were compared using mixed effect models.
The progression of retinal pigment epithelial (RPE) loss was considered the primary endpoint. Secondary endpoints consisted of external limiting membrane (ELM) disruption and ellipsoid zone (EZ) loss. These pathognomonic imaging biomarkers were quantified using validated deep learning algorithms.
“We observed significantly slower disease progression in recently developed cRORA lesions measured on SD-OCT,” the authors explained in their paper for Ophthalmology Retina. “Most importantly,” they wrote, “most relevant signs of future disease progression, like photoreceptor degeneration, are already visible during the conversion from incomplete RORA to cRORA.”
RPE loss and ELM disruption showed a significantly smaller growth rate in eyes with recently developed cRORA lesions compared with patients with advanced cRORA lesions.
The specific cRORA measurement on SD-OCT could lead to smaller growth rates compared with more general GA lesion measurements with other imaging modalities, such as fundus autofluorescence (FAF).
“This effect can be attributed to RPE dysmorphia on the GA lesion edges that do not fulfill the CAM criteria for cRORA but might be identified as part of the lesion on FAF imaging,” the authors explained. “Smaller growth rates for very large cRORA lesions at baseline have been previously described. As we did not have any restriction regarding maximal baseline lesion size during our recruitment, some of the patients in the advanced cRORA study cohort might show slower growth rates due to their large baseline size.”
EZ loss showed no significant difference between the two groups.
“The segmentation of EZ reflectivity and its disruption can be challenging,” the authors wrote. “More data will be needed to accurately interpret the growth rate of EZ loss. In-depth insights into the dynamics of photoreceptor loss in GA warrant attention as recent publications have shown the significant impact of complement inhibitors in slowing photoreceptor decline in GA. Advances in imaging technology might allow a more precise interpretation of the EZ and therefore photoreceptor functionality.”
EZ loss was consistently larger than RPE loss, which is similar to the findings of recent studies describing an outreaching of EZ loss beyond the borders of the cRORA lesion. EZ and photoreceptor loss have previously been described as precursors of future RPE atrophy in GA, the authors noted.
“Subjects in the fresh converter cohort showed significantly higher EZ/RPE loss ratios in all timepoints compared with their counterparts in the advanced study cohort,” they wrote in their article. “We hypothesize that early cRORA lesions, where the pathologic disease-promoting mechanisms are not established completely, already show early signs of disease activity such as a photoreceptor decline. During the natural course of the disease progression, the ratio between RPE loss and EZ loss decreases due to advancing and secondary ‘catching-up’ of the subsequent RPE degeneration. Nonetheless, we need to consider that EZ/RPE loss ratios of recently converted cRORA lesions might still be skewed due to the small lesion size, leading to a higher ratio variability in comparison to more advanced and larger lesions.”
The authors concluded that individual disease monitoring using AI-based quantification paves the way toward optimized GA management.
“OCT imaging should be the preferred imaging modality, as it provides a 3D, in-depth analysis of all retinal layers particularly at the photoreceptor level which represents the functional correlate.”
Coulibaly LM, Reiter GS, Fuchs P, et al. Progression dynamics of early versus later-stage atrophic lesions in non-neovascular AMD using quantitative OCT biomarker segmentation. Ophthalmology Retina. May 3, 2023. [Epub ahead of print.] |


