Editor’s Note: As part of our “Year in Review” retrospective, we’ve selected the top 30 news stories of the year and are re-sharing them as we close out 2022. Follow along as we count down to number 1!
This story was originally published on October 20, 2022.
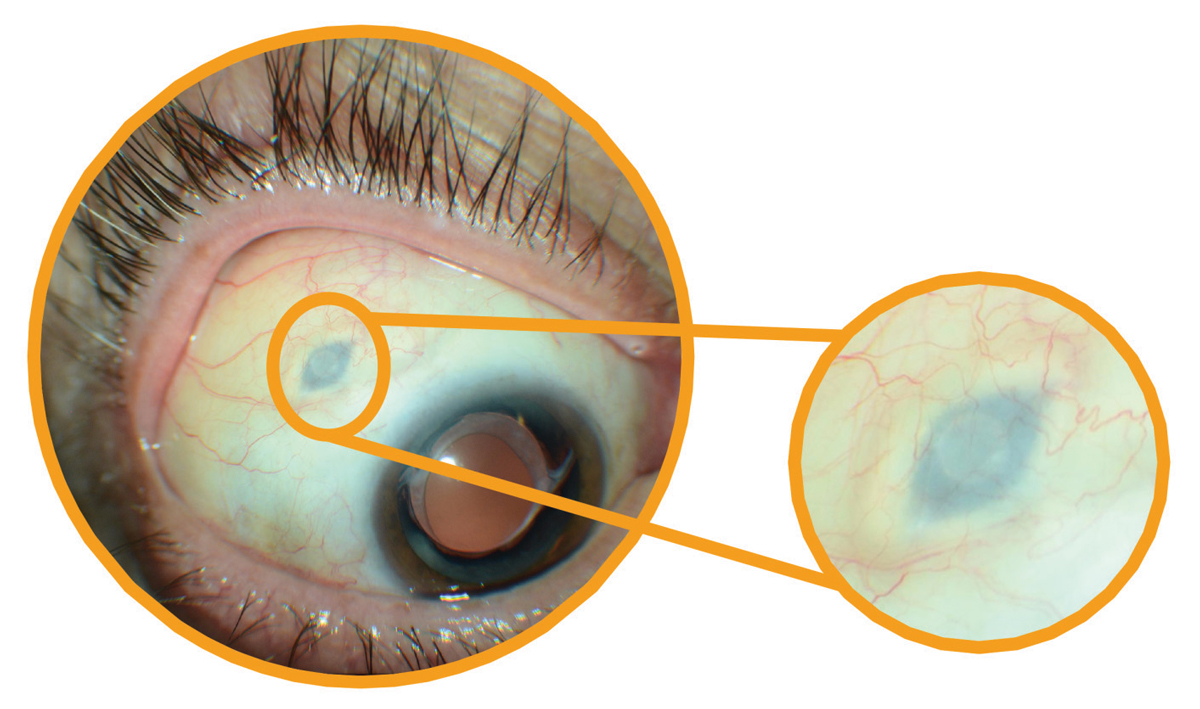 |
|
Patients with the implant do not require explantation and will continue to receive drug refills as needed, Roche explained. Click image to enlarge. |
Around this time last fall, the FDA approved the first sustained-release drug delivery system for wet AMD, the intravitreal implant Susvimo (100mg/mL ranibizumab injection, Genentech/Roche), a novel treatment approach requiring a medication refill only every six months. Fast forward to this year and the product is being pulled from US shelves due to a voluntary manufacturer recall relating to a potential leakage problem.
Between twice-yearly treatments, the implant is designed to dispense the anti-VEGF agent into the vitreous in a controlled manner. However, on Tuesday Roche CEO Bill Anderson explained in an investor call that, due to a manufacturing issue, the company has cause for concern that there may be a problem with the seal on the intravitreal device that’s intended to prevent the medication from leaking out after it’s injected. As reported in the industry publication Fierce Pharma, Mr. Anderson communicated Roche’s concern about the possibility that the seal could fail after repeat dosing and is quoted as saying, “because it didn't meet our performance standards, and [because] we want to make sure that we have high reliability, we decided to voluntarily stop distribution of the port delivery system.”1
Roche advises patients who already have the Susvimo implant to continue receiving refills as normal, and notes that explantation is not necessary. However, no new patients will be able to receive the implant until the production issues are resolved and the device returns to the market, which the company estimates will be approximately within a year or so.
1. Kansteiner F. Roche recalls new eye therapy Susvimo on leakage fears, aims for market return 'within a year or so'. Fierce Pharma. Published October 18, 2022. https://www.fiercepharma.com/manufacturing/roche-recalls-susvimo-implant-lucentis-leakage-fears-return-market-expected-within. Accessed October 19, 2022. |


