Eight years ago, a 56-year-old white female presented to our office as a new patient with complaints of decreased near vision. She reported that her previous eye doctor had followed her “off and on” for “high pressures” in her eyes, but she denied being medicated. Her only medications were HCTZ for hypertension and potassium supplements. She reported that her last visual field test was about five years earlier.

Diagnostic Data
Entering visual acuity at her initial visit was 20/25 O.D., O.S., and 20/20- O.U. Best-corrected visual acuity was 20/15 O.D., O.S., and O.U., and J1 O.D. and O.S. Pupils were round and reactive to light and accommodation with minor physiological anisocoria of 0.5mm. Confrontation fields, as well as extraocular motilities, were full in all positions of gaze.
Slit lamp examination of her anterior segments was unremarkable. Intraocular pressure was 15mm Hg O.D. and 18mm Hg O.S. at 10:30 a.m. Her angles were open and estimated to be grade 4 O.U.
Upon dilation, her crystalline lenses were clear in both eyes. I estimated cup-to-disc ratios of 0.25 x 0.30 O.D. and 0.30 x 0.35 O.S. Her neuroretinal rims were plush and well perfused in both eyes, with no areas of suspect anatomy. Her maculae and vasculature were unremarkable in both eyes. The peripheral retinas had scattered areas of pigmented cystoid in both eyes, but there were no holes, tears or tractional phenomenon. Also, we took stereo-optic nerve photos.
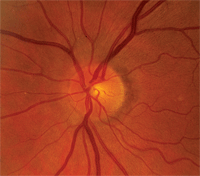
For years, this patient had fluctuating intraocular pressures, but stable optic discs, before converting to glaucoma.
Given the low index of suspicion at this visit, we scheduled the patient to return in one year for standard follow-up. In the interim, we obtained her previous records.
At the one-year follow-up visit, her visual acuity and the remainder of her ophthalmic evaluation were essentially unchanged, including her cup-to-disc ratios and neuroretinal rim status. The only difference: Her IOP was now 20mm Hg O.D. and 21mm Hg O.S.
She came back in two weeks for a full glaucoma workup. At this visit, her threshold visual fields were unremarkable O.U., with moderately good reliability indices. IOP was 22mm Hg O.D. and 24mm Hg O.S. Gonioscopy demonstrated grade 3 to 4 open angles in both eyes, with normal trabecular pigmentation and no angle anatomic abnormalities. Her cup-to-disc ratios were 0.25 x 0.30 O.D. and 0.30 x 0.35 O.S.
Given these findings, I believed that the patient was at risk of developing glaucoma, but she had not yet manifested changes warranting interventional therapy. So, we scheduled her for check-ups every six months.
During the next few years, her IOP varied from 13mm to 23mm Hg O.D. and 16mm to 25mm Hg O.S. Her optic nerves and visual fields remained stable. We also obtained pachymetry measurements: 565µm O.D. and 571µm O.S.
Then, in December 2010, she presented with IOP of 28mm Hg O.D. and 29mm Hg O.S. Topographic Change Analysis (on Heidelberg Retina Tomograph-3) demonstrated early changes to the neuroretinal rims in both eyes. Standard white-on-white perimetry results remained unchanged. Gonioscopy showed open angles with minimal trabecular pigmentation O.U.
Diagnosis and Treatment
In February 2011, her IOP was 27mm Hg O.D. and O.S., and repeat topographic scans confirmed the subtle neuroretinal rim changes. At this point, I was convinced that the patient had converted to glaucoma, so I prescribed 1gtt Lumigan (bimatoprost, Allergan) O.U. h.s.
Two weeks after initiating medication, IOP measured 20mm Hg O.D. and 19mm Hg O.S. A follow-up one month later revealed IOP of 17mm Hg O.D. and 21mm Hg O.S.
At the most recent visit in May 2011, IOP was 19mm Hg O.D. and 17mm Hg O.S. The topographic analysis remained unchanged.
|
|
Discussion
This case highlights two important considerations in the management of your glaucoma patients: fluctuations in IOP before a diagnosis of glaucoma, and fluctuations in IOP after glaucoma diagnosis and the patient is medicated.
The patient in this case presented with a previous history of being watched for elevated IOP, with fluctuations from the low teens to the low 20s. During the years that I monitored the patient, her IOP varied by as many as 10mm Hg, yet her neuroretinal rims remained stable. Eventually her IOP climbed, the neuroretinal rims underwent subtle changes, and she required therapy.
A Simple Plan for Scheduling Patients
In my office, we do our best to try to schedule glaucoma suspects and
glaucoma patients at specific times and intervals to account for
fluctuations in IOP:
• Ocular hypertensives with normal nerves. Two to three times per year,
depending on level of suspicion. I like to see these patients at the
same time of day, each visit, preferably in the morning.
• Glaucoma suspects with suspect nerves and non-elevated IOP. These
typically normotensive suspects are also seen two to three times per
year. IOP fluctuation may play more of a role in these patients, so I
tend to see them at varied times throughout the day.
• Patients who are going to be medicated. Because IOP reduction is the
only modifiable risk factor we have, we try to schedule the patient for
two visits at approximately the same time of day, preferably in the
morning, before beginning a drug or surgery. Once the patient is
treated, we try to schedule their next two visits for about that same
time of day. In this way, we are able to compare pre- to post-treatment
pressures at the same point in the individual’s diurnal curve.
• Long-standing medicated patients. For these patients, we want to
assess their overall stability, which is largely accomplished through
imaging and visual fields. But IOP remains a risk factor that needs to
be considered, so I typically see these patients consistently in the
morning at least for a year or two. Occasionally, I schedule a follow-up
visit as late as possible from their last medication dose (usually an
afternoon appointment) to get a feel for the IOP prior to the next
medication dosage.
The role of IOP fluctuation in the risk of developing glaucoma, as well as its role in the progression of glaucoma, has been the basis of extensive study recently. Much of the evidence indicates that variable IOP does play a role in the risk of developing glaucoma, but that risk cannot be looked at in a vacuum. We must also take into consideration concurrent IOP levels, the amount of optic nerve cupping already present, central corneal thickness, the presence of disc hemorrhages and the presence or absence of field defects.
Likewise, once a diagnosis of glaucoma has been made, IOP fluctuations play a role in the progression of glaucoma, but fluctuations in IOP without careful evaluation of the fields and neuroretinal rim status is only looking at a part of the picture.1,2 One recent study concluded that fluctuation of IOP was not as predictive of field progression as the peak IOP was.3
But clinically, how do you actually measure IOP fluctuations, and how do you schedule your patients? It’s a difficult task to accomplish, especially if you’re in a busy private practice and your scheduling is highly dependent on patient availability.4 Occasionally, it’s feasible to get a diurnal curve on some glaucoma patients, at least from early morning to early evening; but that’s the exception, not the rule. In my office, we try to simplify patient scheduling and, to some extent, normalize the variables. (See “A Simple Plan for Scheduling Patients.”)
While there are several ways to look at IOP throughout the day, this plan works well for me. Of course, many factors can disrupt this schedule. No matter how you schedule your follow-up visits, do it in a consistent manner: Have as many fixed parameters as possible to aim at this constantly moving target.
As for the patient in this case, I’m not convinced that her IOP has stabilized. Only time will tell, but with as few variables as possible interfering with IOP measurement, we will know earlier.
1. Nouri-Mahdavi K, Hoffman D, Coleman AL, et al; Advanced Glaucoma Intervention Study. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004 Sep;111(9):1627-35
2. Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008 Jul;115(7):1123-9.
3. De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011 May;129(5):562-8.
4. Sultan MB, Mansberger SL, Lee PP. Understanding the importance of IOP variables in glaucoma: a systematic review. Surv Ophthalmol. 2009 Nov-Dec;54(6):643-62

