 |
A 61-year-old male presented with blurred distance and near vision in the left eye with occasional dull ache for about one month. He had a history of glaucoma, diabetes mellitus type 2, hypercholesterolemia, and a reported minor stroke 10 years prior. He advised that he was prescribed an eye drop with a teal cap to use in each eye, but admittedly, had not been using it consistently and had been lost to follow-up from the prescribing physician. His pinhole visual acuity was 20/20 OD and 20/400 OS with afferent defect in the left eye. His left eye was diffusely hyperemic with moderate stromal edema, but without microcystic corneal edema. There was neovascularization of the left iris and a rare cell in the anterior chamber, but no hyphema. Intraocular pressures (IOPs) were 30mm Hg OD and 47mm Hg OS.
The right anterior chamber angle was open to posterior trabecular meshwork 360º with 1+ trabecular meshwork pigment and a flat iris approach without peripheral anterior synechiae, angle recession or neovascularization of the angle. In the left anterior chamber angle, anterior trabecular meshwork could be visualized 270º with no structures visible temporally. With compression, neovascularization was present temporal and inferior with peripheral anterior synechiae superiorly. Optic discs were sharp and pink, with inferior notching of the right neuroretinal rim and significant inferior and superior neuroretinal rim loss in the left eye. The right macula was unremarkable. The left macula was diffusely irregular with few intraretinal hemorrhages scattered through the posterior pole and macula. Retinal arterioles were symmetrically attenuated, with increased venular dilation and tortuosity in the left eye. The retinal periphery was unremarkable in each eye.
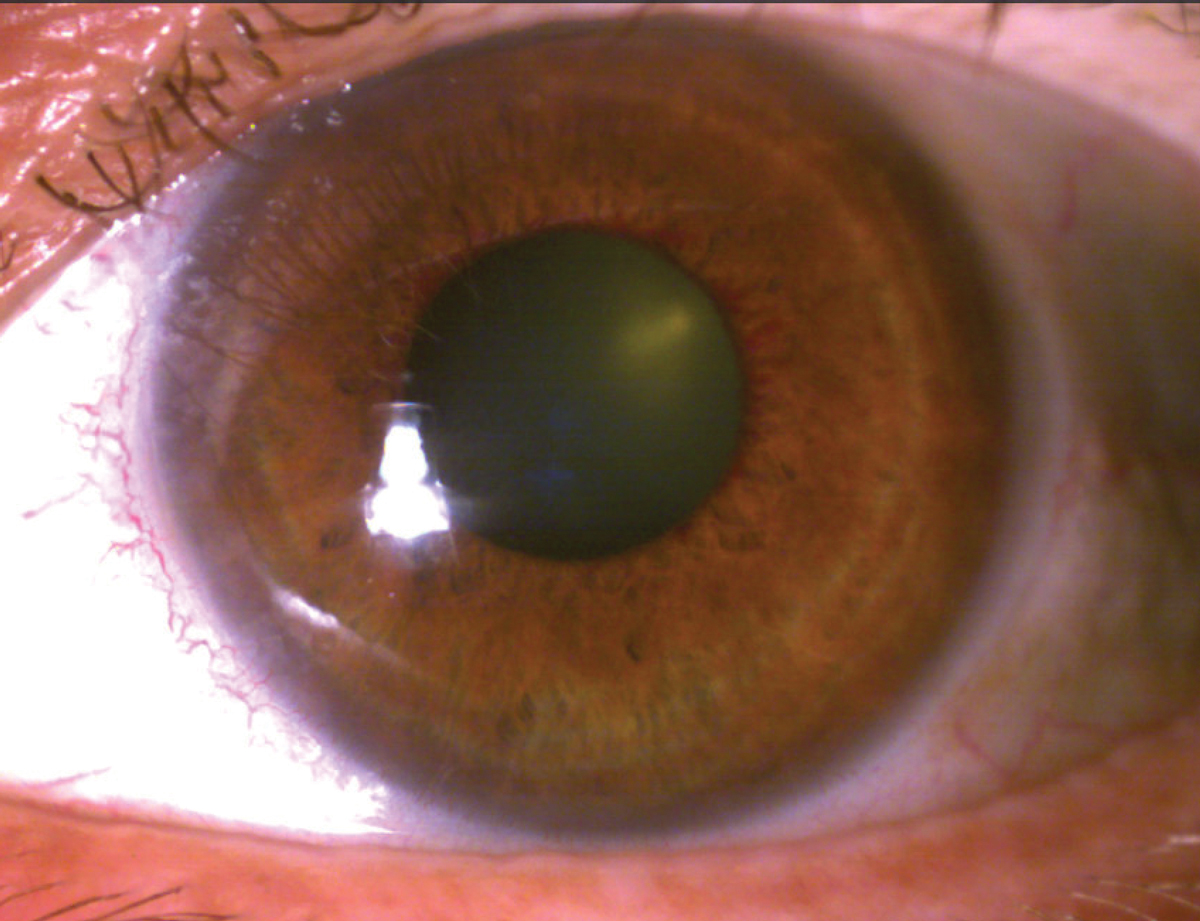 |
|
Peripupillary iris neovascularization. Click image to enlarge. |
Sequelae of Ischemia
Neovascular glaucoma (NVG) is a result of chronic posterior segment ischemia, most commonly caused by proliferative diabetic retinopathy, retinal vaso-occlusive disease and ocular ischemic syndrome.1,2,3 It is estimated to account for between 4% and 6% of all glaucoma diagnoses in Romania and China, with proliferative diabetic retinopathy as its leading cause.4,5 Most patients who present with NVG do so late in the disease course with profound vision loss and ocular pain, but a progressive spectrum of disease exists beginning with early, asymptomatic neovascularization of the iris or angle without elevated IOP.1,2
Iris neovascularization occurs in response to upregulation of a host of growth factors and inflammatory cytokines produced in response to oxidative stress, including vascular endothelial growth factor-A (VEGF-A) and erythropoietin in the anterior chamber due to chronic posterior segment ischemia, which drives new blood vessel formation.1,3,6 Early rubeosis may be visualized at the pupillary margin and base of the iris.
With progression, fibrovascular tissue extends through the anterior chamber angle, across the ciliary body band and trabecular meshwork. At this point, even without secondary angle closure, IOP increases due to reduced aqueous outflow. Increased inflammatory mediators drive synechial angle closure and posterior synechiae formation, and the fibrous scaffold that neovascular vessels use to advance through the angle mature and contract, zipping the angle closed in the process. This ultimately results in further release of pro-inflammatory cytokines, secondary angle closure, significant elevation in IOP and glaucomatous optic neuropathy.1-3
Treatment
Goals of NVG therapy are twofold: to manage IOP and to reduce retinal ischemia and VEGF production.1-3,7 The prognosis of NVG is generally poor with a recent study reporting 74% of patients diagnosed with NVG due to central retinal vascular occlusion had counting fingers or worse vision regardless therapy or combination of therapy through five years of follow-up.7 Considering the advanced optic neuropathy and vision loss often observed at initial presentation, the long-term practical goals of therapy are to prevent or improve severe ocular discomfort and to preserve any remaining visual function.1,2
Initial medical management of NVG is aimed at reducing IOP, as well as improving corneal clarity and patient comfort. IOP-lowering therapies that reduce aqueous production: beta-blockers, carbonic anhydrase inhibitors and alpha adrenergic agonists are generally our first-line choices, often in combination due to the significant IOP-lowering effect called for.1-3 Despite their pro-inflammatory potential, prostaglandin analogs can also be considered as adjunctive therapy and have been demonstrated to have clinical effect in eyes with even complete synechial angle closure.8 Pilocarpine should be avoided due to its ability to exacerbate angle closure and increase inflammation.1 A strong topical steroid, prednisolone acetate 1% or difluprednate 0.05%, and a strong cycloplegic agent, atropine 1%, will help reduce inflammation and prevent ciliary spasm to improve comfort.1,3
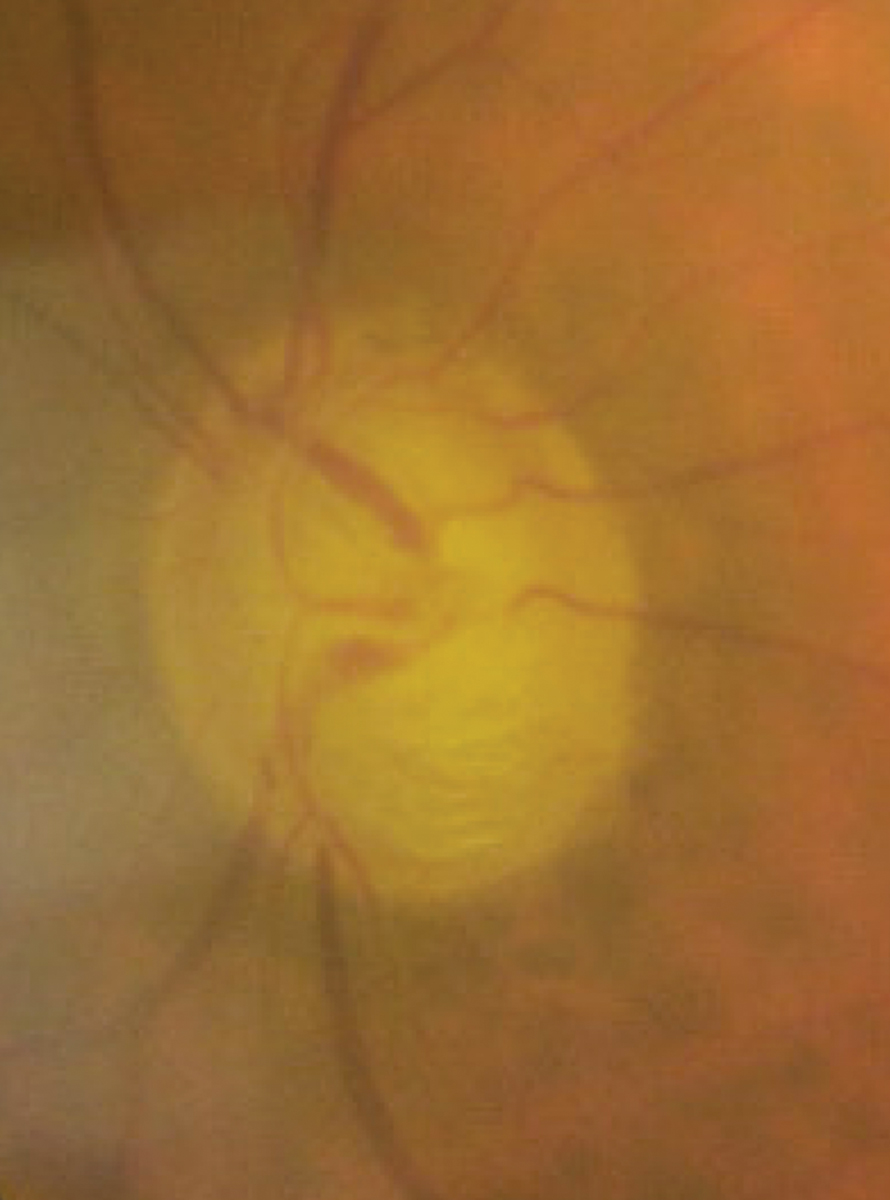 |
|
Advanced glaucomatous optic neuropathy due to NVG two months following initial presentation. Click image to enlarge. |
Panretinal photocoagulation (PRP) aims to reduce ischemic stress and production of pro-angiogenic proteins through destruction of retinal tissue.1,6 Clear media is a requirement for PRP, and in individuals with vitreous hemorrhage or who have significant corneal edema, PRP will not be pursued until the media clears. While an effective therapy in reducing oxygen demand, and therefore oxidative stress, regression of abnormal new vessels may take four to six weeks, leaving a window for further disease progression and vision loss without adjunctive therapy.6
Anti-VEGF therapies in NVG treatment have shown to regress iris neovascularization and reduce IOP within days of injection, but have a short duration of action.6 Aqueous samples of patients with NVG contained approximately three-fold higher VEGF-A levels, and two-fold higher IL-8 levels in patients with newly diagnosed NVG in comparison to those following treatment (PRP, with or without intravitreal bevacizumab) and who were determined to be clinically stable.6 VEGF-A and IL-8 levels were found to be positively correlated with IOP; that is, in NVG, eyes with higher IOP had higher levels of aqueous VEGF-A and IL-8.6 Due to the central mechanistic but temporary effect of anti-VEGF agents in NVG management, they are most likely currently provided as initial retinal therapy for neovascular regression and are followed by PRP or other surgical procedures.9,10
Once the source of the retinal ischemia and underlying systemic risk factors have been managed, long-term treatment shifts back to managing IOP to preserve any remaining functional vision and to reduce ocular discomfort. Topical therapy alone may not be sufficient, and incisional filtration or aqueous shunt procedures may be necessary. For patients who have no visual potential and severe ocular discomfort, retrobulbar alcohol injection or enucleation are additional strategies that reflect the severity of the condition and the profound long-term discomfort which individuals may experience.1
Bottom Line
Based on the NVG diagnosis of the left eye secondary to presumed retinal vascular occlusion and primary open-angle glaucoma of the right eye, our patient was prescribed dorzolamide-timolol fixed combination BID in each eye and latanoprost 0.005% QHS in each eye along with prednisolone acetate 1% QID OS and atropine 1% QD OS. Next-day retinal consultation was arranged, as was follow-up with his internist and a plan for continued care in our office of his primary open-angle glaucoma. At his initial visit with a retinal specialist approximately one week later, he received an intravitreal bevacizumab injection and was scheduled for PRP.
Identification of risk factors for the development of NVG, early recognition of anterior segment neovascularization and prompt, aggressive therapy and coordination of care is the core to preserving vision, where possible, and limiting ocular discomfort associated with the disease process and its underlying cause.
Dr. Steen is an associate professor at Nova Southeastern University College of Optometry where she serves as director of the Glaucoma Service, coordinator of the Primary Care with Emphasis in Ocular Disease Residency and teaches courses in glaucoma and ocular pharmacology. Her financial disclosures include Bausch & Lomb, Santen, Ocuphire and Carl Zeiss Meditec.
1. Sivak-Callcott JA, O’Day DM, Gass JD, et al. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108(10):1767-76. 2. Havens SJ, Gulati V. Neovascular glaucoma. Dev Ophthalmol. 2016;55:196-204. 3. Tang Y, Shi Y, Fan Z. The mechanism and therapeutic strategies for neovascular glaucoma secondary to diabetic retinopathy. Front Endocrinol (Lausanne). 2023;14:1102361. 4. Liao N, Li C, Jiang H, et al. Neovascular glaucoma: a retrospective review from a tertiary center in China. BMC Ophthalmol. 2016;16:14. 5. Mocanu C, Barăscu D, Marinescu F, et al. Glaucomul neovascular--studiu clinic retrospectiv [Neovascular glaucoma--retrospective study]. Oftalmologia. 2005;49(4):58-65. 6. Sun C, Zhang H, Tang Y, et al. Aqueous inflammation and ischemia-related biomarkers in neovascular glaucoma with stable iris neovascularization. Curr Eye Res. 2020;45(12):1504-13. 7. Casselholm de Salles M, Lindberg C, Epstein D. Neovascular glaucoma in patients with central retinal vein occlusion: a real-life study in the anti-VEGF era. Acta Ophthalmol. 2021;99(1):e7-12. 8.Vyas P, Naik U, Gangaiah JB. Efficacy of bimatoprost 0.03% in reducing intraocular pressure in patients with 360° synechial angle-closure glaucoma: a preliminary study. Indian J Ophthalmol. 2011;59(1):13-6. 9. Rittiphairoj T, Roberti G, Michelessi M. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst Rev. 2023;4(4):CD007920. 10. Ramji S, Nagi G, Ansari AS, et al. A systematic review and meta-analysis of randomised controlled trials in the management of neovascular glaucoma: absence of consensus and variability in practice. Graefes Arch Clin Exp Ophthalmol. 2023;261(2):477-501 |

