 |
Q:
Many of my patients with AMD need monthly injections. I just heard about the new drug Vabysmo (faricimab-svoa, Genentech) that extends this to every four months. What can I tell my patients?
In the last nearly two decades intravitreal injections of anti-VEGF have become standard in treatment of neovascular (or wet) AMD, diabetic retinopathy, diabetic macular edema and retinal vein occlusion. Agents such as Lucentis (ranibizumab, Genentech) and Eylea (aflibercept, Regeneron) have shown safety and efficacy in clinical trials, and as a result, have received FDA approval for several years. Avastin (bevacizumab, Genentech) is a good inexpensive alternative and has been used frequently as a non-FDA approved agent.
“Although these remedies are effective in addressing a variety of conditions, there are pitfalls associated with the drug’s duration as well as mechanism of action,” says Mohammad Rafieetary, OD, of Charles Retina Institute in Germantown, TN. “The short durability of these agents results in the need for frequent injections, often once per month in many cases.”
The need for repeated injections can cause several burdens and barriers, which include injection anxiety and fatigue, access to care and transportation and cost, especially to the uninsured. These factors lead to poor compliance and adherence with the treatment regimen and, subsequently, poor outcomes. Another issue is partial effectiveness. The biologic processes such as angiogenesis and vascular permeability are not just VEGF driven. There are other biological systems and chemicals, including different growth factors such as angiopoietins and platelet-derived growth factor, that can lower the effectivity of the drug. These issues have prompted a surge in research and development of alternative therapies, such as novel molecules, dosing variations and different routes of administration.
 |
|
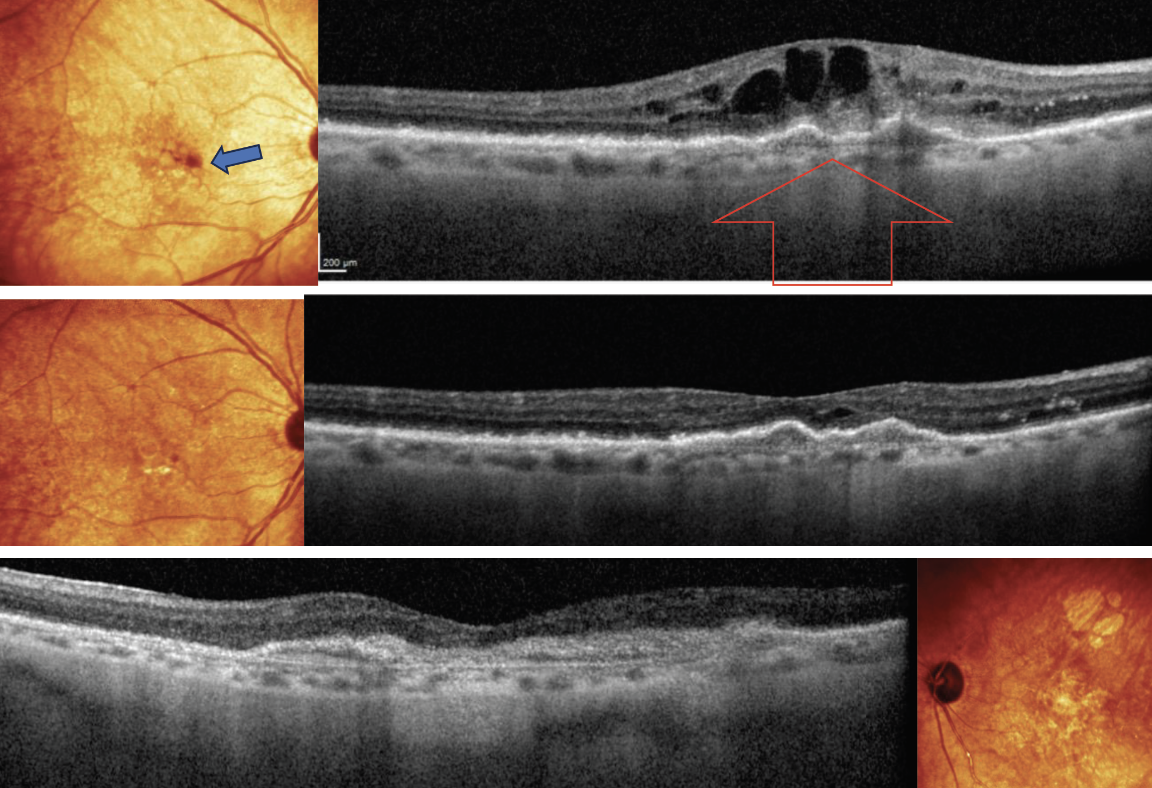
OD fundus exam shows small subretinal hemorrhage (blue arrow) and OCT (top) is remarkable for choroidal neovascularization (CNV), as shown with red arrow. OS has a large disciform scar (bottom image). Patient received an intravitreal injection of Vabysmo. At one month follow-up, regression of CNV was noted (middle image) and VA has improved to 20/30. At this time, patient receives her second injection, with her next appointment set for three months. Click image to enlarge. |
Two for One Deal
Vabysmo (faricimab, Genentech) is a biphasic agent, meaning that it has two distinct components each with a different mechanism of action. This drug targets VEGF-A and the Tie-2/angiopoietin pathway. The STAIRWAY and AVENUE studies were Phase II clinical trials that showed its efficacy for treatment of wet AMD. The BOULEVARD trial showed its superiority to monthly injections of Lucentis for DME.
Because of that, the FDA has approved the following regimens: four loading doses of 6mg (0.05mL) every four weeks (q28 days), followed by OCT and visual acuity evaluation at eight and 12 weeks, followed by a 6mg dose given in one of the following three regimens: (1) weeks 28 and 44; (2) weeks 24, 36 and 48; or (3) weeks 20, 28, 36 and 44.
“Although additional benefit was not noted in the every four week group as compared with every eight week dosing, some patients may require monthly treatment based on disease activity,” Dr. Rafieetary noted.
According to him, this extended treatment strategy may improve treatment adherence by reducing the burden of frequent visits. Based on these studies and benefits, many retina specialists are switching their patients from traditional anti-VEGF to this new generation biphasic therapy when clinically appropriate and when insurance coverage is available.
“The development of novel agents and increased availability of pharmaceutical agents such as Vabysmo will improve compliance and the quality of life of patients with chronic retinal conditions such as wet AMD and DME,” Dr. Rafieetary concludes. “Know what your retinal surgeon is doing so that you can properly advise patients. Some retinal specialists may not adopt this new modality as quickly as others.”
Dr. Ajamian is board certified by the American Board of Optometry and serves as Center Director of Omni Eye Services of Atlanta. He is vice president of the Georgia State Board of Optometry and general CE chairman of SECO International. He has no financial interests to disclose.
1. Nicolò M, Ferro Desideri L, Vagge A, Traverso CE. Faricimab: an investigational agent targeting the Tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021;30(3):193-200. |

