 |
The macula is a small, highly-specialized area of the retina that is responsible for providing our sharpest acuity. Its unique anatomical and biochemical features distinguish it from the rest of the neighboring as well as peripheral retina and contribute to its susceptibility to certain ocular disease processes. When we explore the details of this region, we are better able to understand the pathogenesis of many macular conditions.
Anatomical and Biochemical Features
The macula is approximately 5mm to 5.5mm in size and is further subdivided into a central 0.35mm fovea. Additional surrounding areas include the parafovea and perifovea. Note that the highest density of cone photoreceptors is located in the fovea, approximating 50 cone cells per 100µm and resulting in the ability to perceive sharp visual detail. The fovea is also uniquely composed of fewer layers than the rest of the neurosensory retina, limited only to the thin inner plexiform layer, outer nuclear layer, photoreceptors and retinal pigment epithelium (RPE). This alteration allows for the tight packing of cones in this space. As we move away from the fovea and to the more peripheral areas of the macula, both cones and rods begin to occupy the space.1
Directly adjacent to the RPE layer is the underlying choriocapillaris, which is the primary vascular supply of the macula. Within the innermost macula, there is a lack of vasculature, termed the foveal avascular zone. This is a unique distinction in that the rest of the retina is supplied by the central retinal artery system and underlying choroid.1,2 Despite the lack of vasculature, the macula exhibits a high degree of metabolic activity. Light is continuously being synthesized to vision, requiring a substantial amount of oxygen. As such, the retina is one of the highest oxygen-consuming tissues in the body.2
Clinical Applications
Given these characteristics alone, the macular region is more susceptible to certain disease processes that do not normally occur otherwise throughout the remaining retinal anatomy. If we apply the principles mentioned above, we are able to better understand the pathophysiology as it correlates to various conditions.
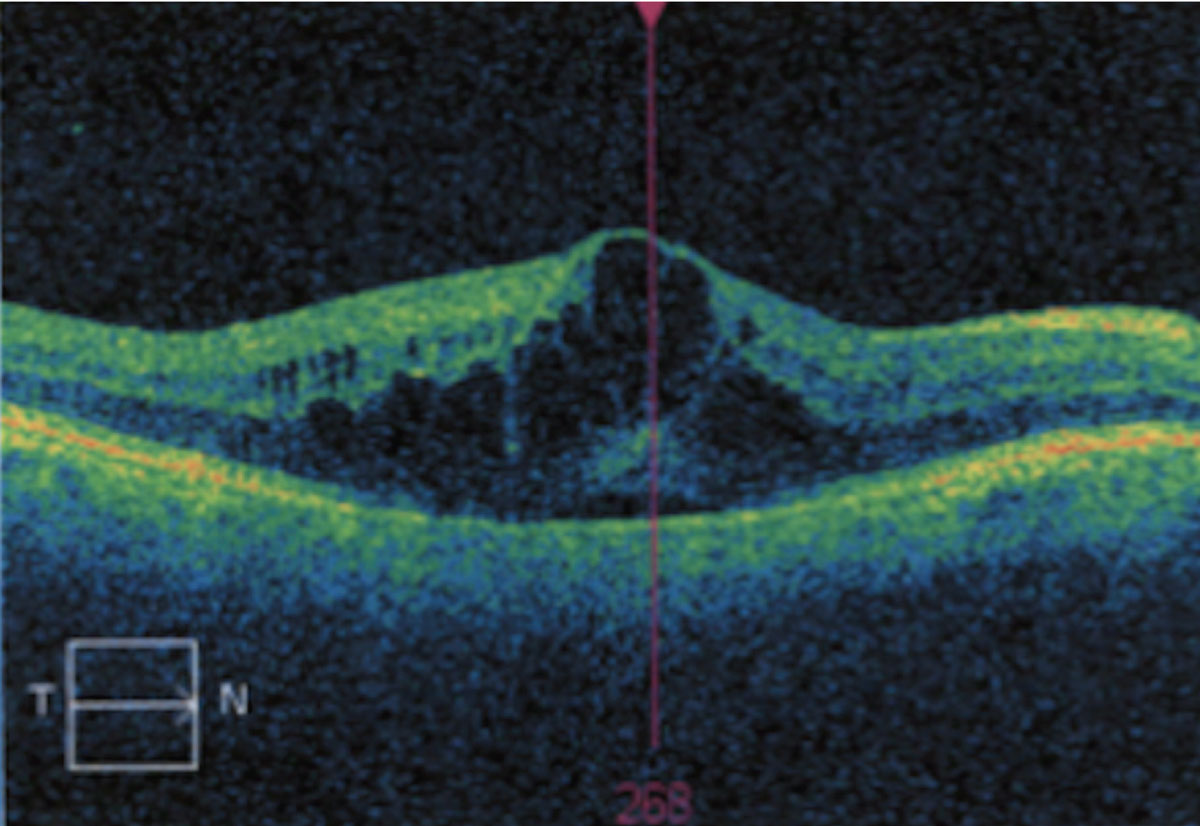
Cystoid macular edema (CME). This condition is generally caused by a breakdown of the blood-retinal barrier and the resultant thickening from fluid accumulation within the neurosensory retina. This manifestation may occur from primarily ocular conditions or secondary to systemic disease conditions. Examples of such cases are uveitis, diabetes, vein occlusion, retinitis pigmentosa, vitreomacular traction or following cataract extraction.3 Because the blood-retinal barrier is compromised, fluid leaks from across the retinal vessels and RPE into the perifoveal tissues.2
 |
|
Cystoid macular edema resulting from fluid accumulation. Click image to enlarge. |
Histological studies have suggested the presence of fluid is most common in the outer plexiform layer of the fovea, as well as Henle’s layer. Cystic spaces have been noted to be greatest in the outer plexiform layer. While it is unclear why the leakage is confined to the macula, it has been suggested that it is due to the high metabolic activity and avascularity in the foveal region, which prevents adequate resorption of fluid. It is also speculated that, since the internal limiting membrane is thinnest over the fovea, there is less barrier to prevent the diffusion of inflammatory mediators.3,4
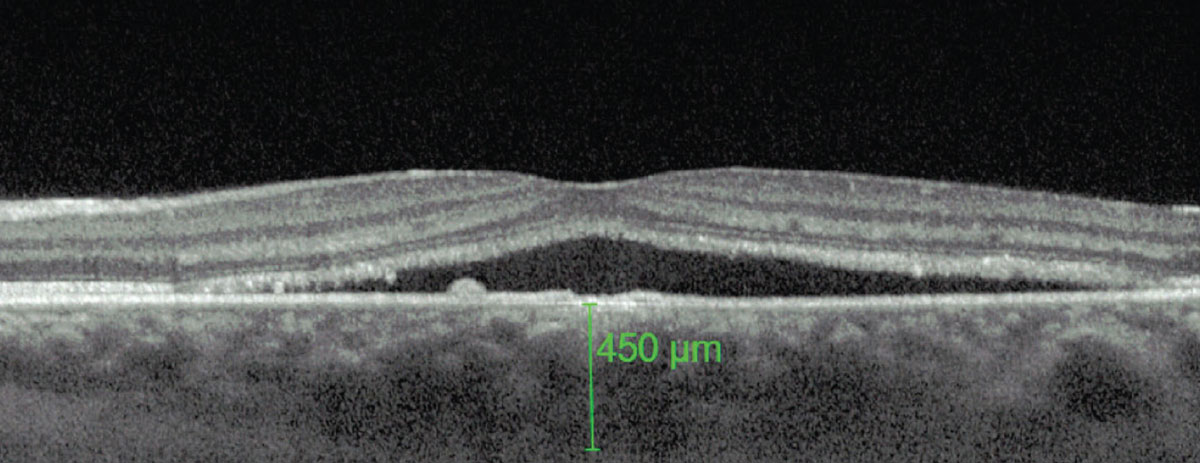
Central serous chorioretinopathy. Recent studies using indocyanine green (ICG) angiography have demonstrated multifocal areas of choroidal vascular permeability secondary to ischemia, stasis or inflammation, suggesting that the choroid is the primary site of pathology.5,6 In patients with this type of chorioretinopathy, increased choroidal thickness has been reported. This variability in size is due to the dilatation of large, hyperpermeable choroidal blood vessels.
Superficial to this thickened layer is an area of medium- and smaller-sized blood vessels known as the inner choroidal layer. In regions where there is marked choroidal thickening, the adjacent inner choroidal layer is thinner than normal tissue due to primary atrophy or direct compression by the underlying dilated vessels. This direct compression and resultant mechanical stress can also lead to reduced RPE adhesion, alteration of RPE hydroionic regulation and RPE atrophy. A combination of these events will ultimately manifest clinically as a pigment epithelial detachment.6 Since the fovea lacks vasculature and is composed of fewer layers, it is more susceptible to choroidal permeability.
 |
|
A thicker-than-average choroid is characteristic of CSCR. Photo: Mohammad Rafieetary, OD. Click image to enlarge. |
Macular glaucoma. Standard and routine glaucoma testing involves the use of optic nerve OCT to measure retinal nerve fiber layer thickness around the optic nerve head along with a VF 24-2 to test function. However, because the macula contains 30% to 50% of the total retinal ganglion cells (RGCs), scanning this area allows a sampling of the majority of the cells implicated in glaucoma.7,8
The ganglion cell and nerve fiber layers constitute 30% to 45% of the total thickness of the macula. Even though the macula, typically defined as the central eight degrees from the foveal center, represents approximately 2% of the retina, it still contains half of all RGCs. In addition, the macula does not contain blood vessels as the optic nerve head does, making it a simpler structure to analyze. Increasing evidence suggests that macular damage is manifest in early or mild glaucoma, where it was once previously thought to be characteristic of advanced stages. To detect such damage using OCT, the RGC and inner plexiform layer (RGC+) should be analyzed.8
Full-thickness macular hole. Macular holes are thought to be a result of anteroposterior traction from the vitreous. As mentioned earlier, the internal limiting membrane in the region of the fovea and macula is thinner than in the rest of the retina. As the membrane is in direct contact with the posterior vitreous cortex, tractional forces would impact this area to a greater degree.9
Age-related macular degeneration (AMD). While this is a complex and multifactorial condition, it is well-established that the macula is a highly metabolic area. It also contains the highest cone density, requiring phagocytosis of outer segments by the RPE. This profound task leads to oxidative stress on the macula, one of the key pathogenic features of AMD development.2
Without the knowledge of the anatomy and biochemistry of the macula as it differs from the other parts of the retina, many macular conditions are not readily understood. In fact, diseases of the macula which were once thought to be idiopathic or of unknown etiology are better recognized today with advancements in technology that allow for more precise examination of this highly specialized area.
Dr. Labib graduated from Pennsylvania College of Optometry, where she now works as an associate professor. She completed her residency in primary care/ocular disease and is a fellow of the American Academy of Optometry and a diplomate in the Comprehensive Eye Care section. She has no financial interests to disclose.
1. Rehman I, Mahabadi N, Motlagh M, Ali T. Anatomy, head and neck, eye fovea. StatPearls. Treasure Island (FL): StatPearls Publishing. 2023. 2. Ruan Y, Jiang S, Gericke A. Age-related macular degeneration: role of oxidative stress and blood vessels. Int J Mol Sci. 2021;22(3):1296. 3. Yilmaz T, Cordero-Coma M, Gallagher MJ. Ketorolac therapy for the prevention of acute pseudophakic cystoid macular edema: a systematic review. Eye (Lond). 2012;26(2):252-8. 4. Ray S, D’Amico DJ. Pseudophakic cystoid macular edema. Semin Ophthalmol. 2002;17(3-4):167-80. 5. Kim HC, Cho WB, Chung H. Morphologic changes in acute central serous chorioretinopathy using spectral domain optical coherence tomography. Korean J Opthalmol. 2012;26(5):347-54. 6. Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58(2):103-26. 7. Sung KR, Wollstein G, Kim NR, et al. Macular assessment using optical coherence tomography for glaucoma diagnosis. Br J Ophthalmol. 2012;96(12):1452-5. 8. Hood DC, De Cuir N, Blumberg DM, et al. A single wide-field OCT protocol can provide compelling information for the diagnosis of early glaucoma. Transl Vis Sci Technol. 2016;5(6):4. 9. Premi E, Donati S, Azzi L, et al. Macular holes: main clinical presentations, diagnosis, and therapies. J Ophthalmol. 2022;2022:2270861. |

