
A 54-year-old white male presented complaining of blurred vision in his right eye for the past four days. When he worked on his computer, he said, it seemed as though he was looking through a glass of water. He was concerned that he might have a retinal detachment, so he came in for an evaluation. He reported no complaints in his left eye.
 |
|
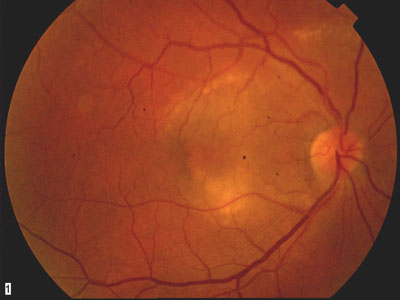
1. A prominent lesion involves the macula in our patients right eye. |
The patient has been HIV+ since 1998. He takes Sustiva (efavirenz, Bristol-Myers Squibb) and Truvada (emtricitabine and tenofovir disoproxil fumarate, Gilead Sciences) for his HIV. He reports that his CD4 count is in the 700 range and that the viral load is undetectable. He also began taking Wellbutrin XL (bupropion, GlaxoSmithKline) six weeks ago to help him quit smoking.
 |
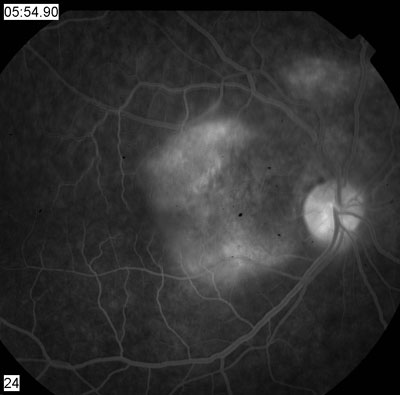 |
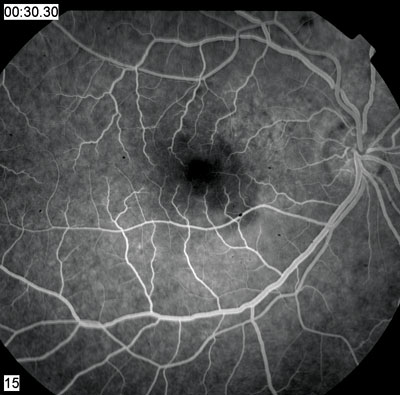
| 2, 3. Early and late frames (30 seconds, left; 54 minutes, right) of the fluorescein angiogram showed these changes. | |
The patient reported a flu-like illness about two weeks earlier, for which he took the Zithromax Z-Pak (azithromycin, Pfizer). He said he was almost fully recovered.
Best-corrected visual acuity measured 20/40 O.D. and 20/20 O.S. Extraocular motility testing was normal. Confrontation visual fields were full to careful finger counting O.U., and his pupils were equally round and reactive with no afferent pupillary defect. The anterior segment exam was unremarkable. IOP measured 15mm Hg O.U.
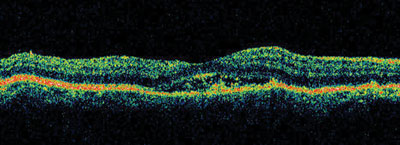
4. Stratus OCT of our patients macula confirms the presence of a neurosensory detachment.
Dilated fundus exam revealed a clear vitreous and small cups with good rim coloration and perfusion in both eyes. Examination of the macula in the right eye revealed the changes seen in figure 1. The fundus exam of the left eye was completely normal. Fluorescein angiography was performed (figures 2 and 3) as well as Stratus optical coherence tomography (OCT)-3 (figure 4) and Spectral Domain OCT (figure 5).
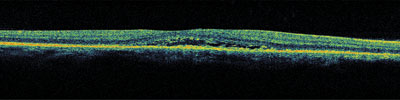
5. Spectral Domain OCT shows high resolution of the macula. Note the changes within the subretinal space.
Take the Retina Quiz
1. Which best describes the staining pattern seen on the angiogram?
a. Early hyperfluorescence building in intensity, late active leakage.
b. Early blockage, late staining.
c. Mottled hyperfluorescence, late leakage and pooling in the macula.
d.
2. What does the OCT show?
a. Small pigment epithelial detachment (PED).
b. Subretinal fluid under the macula with opacities in the subretinal space.
c. Choroidal neovascularization.
d. Retinal thickening secondary to cystoid macular edema.
3. What do the yellow changes in the fundus photo represent?
a. Retinal pigment epithelial detachments (PEDs).
b. Depigmentation of the retinal pigment epithelium (RPE).
c. Photoreceptor outer segments.
d. Inflammation of the RPE.
4. What is the correct diagnosis?
a. Atypical idiopathic central serous chorioretinopathy (ICSC).
b. Choroidal neovascularization.
c. Acute syphilitic placoid chorioretinopathy.
d. Cytomegalovirus.
5. How should this patient be managed?
a. Close observation.
b. Avastin (bevacizumab, Genentech) or Lucentis (ranibizumab, Genentech).
c. Photodynamic therapy (PDT).
d. Oral steroids.
For answers, see below.
Discussion
Our patient has an interesting fundus appearance. On clinical examination, there was shallow subretinal fluid within the macula that appeared to extend outward. There appear to be subtle demarcation lines along the edges of the lesion, where the yellow changes are more prominent. These likely represented an atypical form of ICSC.
Fluorescein angiography and OCT confirm that ICSC is the likely diagnosis. The angiogram shows mottled hyperfluorescence, particularly superior and nasal to the fovea. There is late staining and pooling of the dye in this region in the later frames of the angiogram.
This is not the typical staining pattern of ICSC. The more typical staining pattern: a small or localized area of hyperfluorescence (a hot spot) early in the angiogram that builds in intensity and pools within the subretinal space. The early hyperfluorescence corresponds to a small RPE detachment.
Our patient clearly does not have that. But, in chronic cases of ICSC or in patients whose disease is more widespread, the extent of the RPE disruption can increase. These patients may exhibit more diffuse mottling of the RPE, as seen in our patient. The late staining and pooling are also typical of chronic cases.
The Stratus OCT-3 confirms the presence of a shallow neurosensory detachment. The fluid does not extend too far beyond the fovea. Because our patient was atypical, we also performed Spectral Domain OCT, which showed the shallow subretinal fluid in higher resolution.
We also observed precipitates within the subretinal space. These may represent fibrin, as some patients with ICSC demonstrate a fibrin response. Studies suggest that what was once thought to be fibrin might really be fragments of the photoreceptor outer segments that accumulate when serous detachment of the retina disrupts normal phagocytosis of these segments.1 This is more commonly seen in chronic cases of ICSC.
We suspect the yellow changes in our patients lesion represent accumulation of the photoreceptor outer segments. They do not appear to be RPE detachments, since they do not appear on OCT or the angiogram. They also do not appear to be active RPE inflammation, as there are no vitreous cells and no blockage on fluorescein angiography. The angiogram would likely show blockage in cases of active syphilitic chorioretinopathy or possibly such diseases as acute multifocal posterior placoid epitheliopathy.
Spectral Domain OCT is the latest in OCT technology. The image acquisition speeds are approximately 50 to 100 times faster than standard-resolution OCT systems, and Spectral Domain offers a higher resolution (3m to 6m) vs. traditional OCT (10m).2 The higher resolution may allow for detection of more subtle changes within the retina and individual layers.
We believe that our patient has had ICSC for quite some time, given the changes that are seen clinically and with ancillary testing. The disease process likely has occurred outside his fovea, which would explain the lack of prior symptoms. The recent fluid accumulation under his fovea made him aware that he had an eye problem.
Observation is recommended for patients who have ICSC, as most cases spontaneously improve within six months. For patients whose conditions do not improve or who cannot tolerate the symptoms, laser photocoagulation of the RPE defect is recommended. This does not work well in patients with more widespread RPE disruption, such as our patient.
In these instances, photodynamic therapy (PDT) with Visudyne (verteporfin, Novartis Ophthalmics) has been successful.3
We elected to observe our patient to see if his condition would improve on its own. If it doesnt, he will be a candidate for PDT.
1. Wang M, Sander B, la Cour M, Larsen M. Clinical characteristics of subretinal deposits in central serous chorioretinopathy. Acta Ophthalmol Scand 2005 Dec;83(6):691-6.
2. Srinivasan VJ, Wojtkowski M, Witkin AJ, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 2006 Nov;113(11):2054.e1-14.
3. Taban M, Boyer DS, Thomas EL, Taban M. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol 2004 Jun;137(6):1073-80.
Retina Quiz Answers: 1) c; 2) b; 3) c; 4) a; 5) a.

