 |
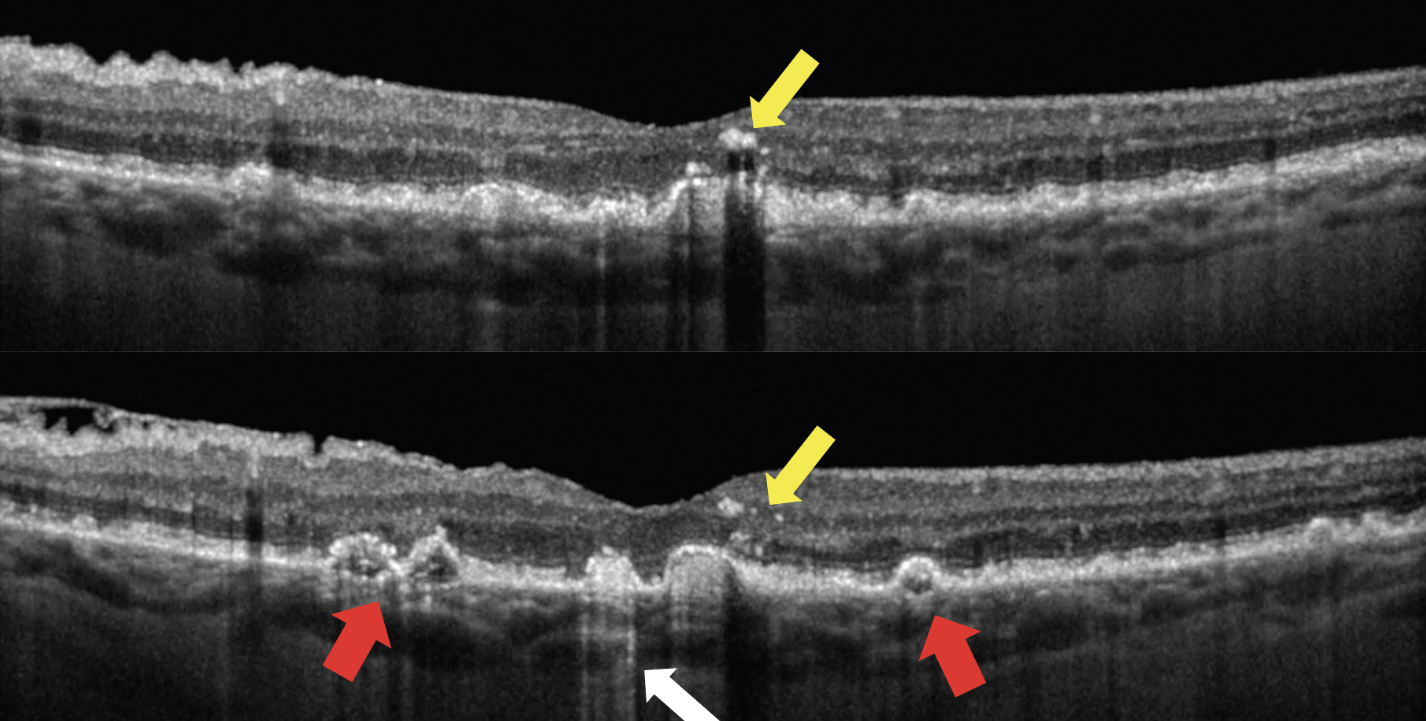
| The distribution of intraretinal hyperreflective foci—not just their presence—is the key driver associated with the risk of progression. The two OCT scans above show a patient with hyperreflective foci (yellow arrows); drusen of irregular, non-uniform internal reflectivity (red arrows); and hyperreflective columns (white arrow). Photo: Jessica Haynes, OD, and Mohammad Rafieetary, OD. Click images to enlarge. |
Intraretinal hyperreflective foci on OCT have been increasingly implicated as one of the most important predictors for progression to late-stage age-related macular degeneration (AMD). These OCT biomarkers have been defined as discrete, > 3-pixel sized, well-circumscribed hyperreflective lesions within the neurosensory retina, having a reflectivity at least comparable to the retinal pigment epithelium band. Understanding their prevalence, distribution and evolution of these lesions over time would appear to be of importance. A recent study assessed the relationship between the distribution of intraretinal hyperreflective foci and progression of intermediate AMD over two years and determined that the risk of progression to late AMD over two years is significantly increased with the number of intraretinal hyperreflective foci in the outer retinal layers.
The Amish Eye Study provided a useful resource for this study of AMD biomarkers over time as its multimodal imaging data was collected prospectively at baseline and two years using a standardized protocol. Among 120 eyes from 71 patients with intermediate AMD, 52 eyes (43.3%) of 42 patients had evidence of both intermediate AMD and intraretinal hyperreflective foci at baseline. The mean age of these patients at baseline was 72.27, and 50.7% were female. The intraretinal hyperreflective foci were counted in a series of five sequential en face slabs from outer to inner retina. The number of intraretinal hyperreflective foci in each slab at baseline and the change in intraretinal hyperreflective foci from baseline to year two were correlated with progression to late AMD at two years.
This study found that 19.0% showed progression to late AMD after two years. The total intraretinal hyperreflective foci count increased from 243 at baseline to 604 at two years, with a significant increase in the intraretinal hyperreflective foci number in each slab, except for the innermost slab 5. The risk of progression to late AMD over two years is significantly increased with the number of intraretinal hyperreflective foci in slab 1 (OR = 2.13) and 2 (OR = 1.46), spanning from roughly the inner border of outer nuclear layer to the outermost portion of the neurosensory retina.
“The lack of an association between intraretinal hyperreflective foci,” the researchers wrote in their paper, “may suggest that intraretinal hyperreflective foci located in the inner retina may be distinct and of a different source of origin than intraretinal hyperreflective foci in the outer retina.”
The team ultimately concluded that “the cellular origin of intraretinal hyperreflective foci at different levels of the retina warrants further study.”
Verma A, Corradetti G, He Y, et al. Relationship between the distribution of intraretinal hyperreflective foci and the progression of intermediate age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. August 11, 2023. [Epub ahead of print]. |

