 |
A 38-year-old African-American female presented to the eye clinic for a comprehensive eye exam with a complaint of blur while reading. During the history, she explained that she was using hydroxychloroquine 400mg daily by mouth for systemic lupus erythematosus control. Her systemic history also included hypertension and anemia, for which she was properly medicated. She reported no allergies to medicines or environmental factors.
Clinical Findings
Her best-corrected entering visual acuities were 20/20 at distance and near through +1.00/+2.25 spectacles, both eyes. External testing was normal, with no defects on confrontation fields or facial Amsler grid and no afferent pupil defect. Anterior segment evaluation was normal and Goldmann applanation tonometry measured 15mm Hg OD and 14mm Hg OS. The pertinent fundus findings are demonstrated in the photographs.
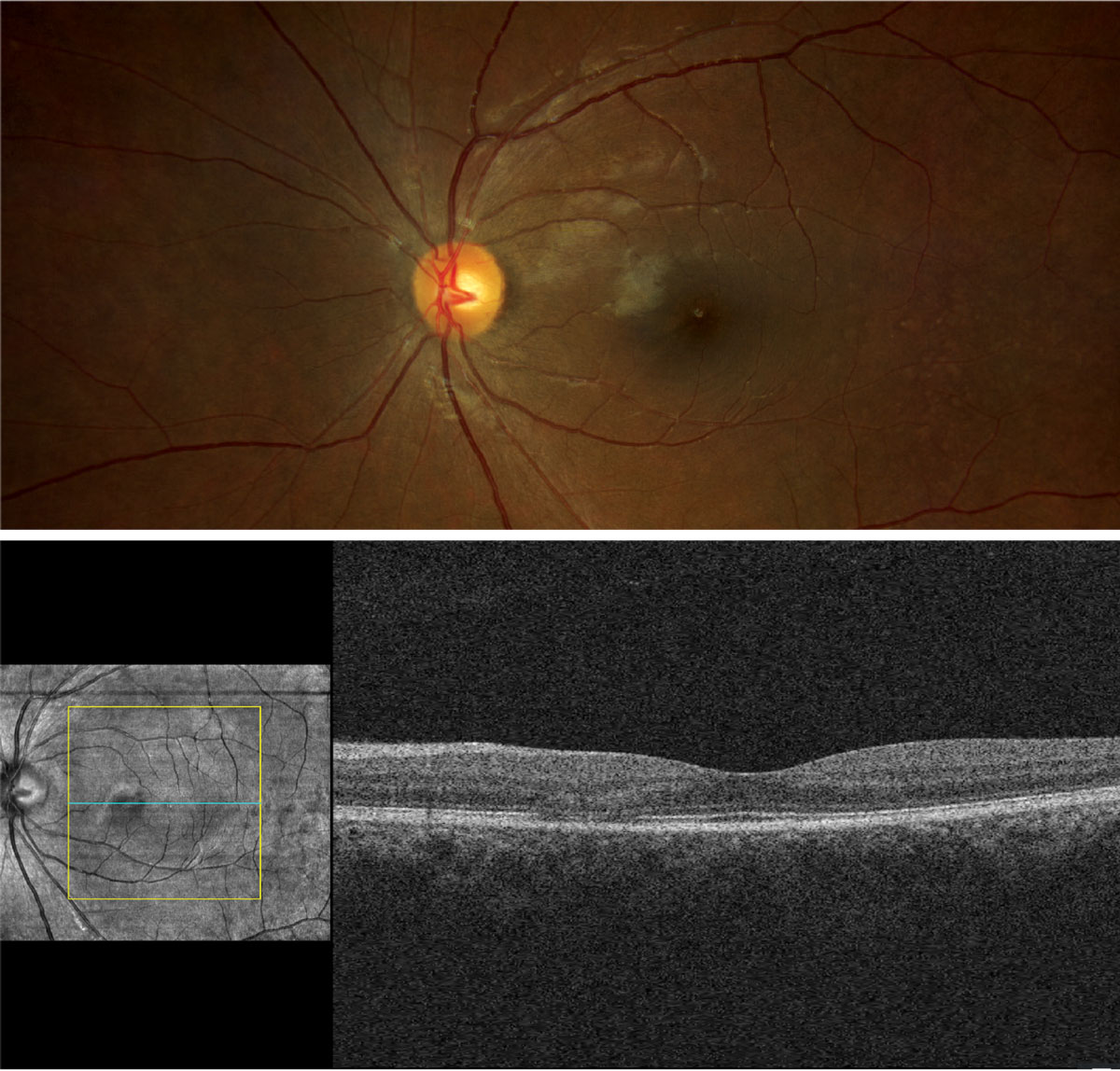 |
|
Does the appearance of the retina confirm any suspicions raised by the case history? Click image to enlarge. |
Additional Testing
Other possible studies might include fundus autofluorescence, fundus photodocumentation and automated (white and red sensitive) perimetry. If the acuity were reduced, laser interferometry may also be useful.
Discussion
The diagnosis in this issue is early hydroxychloroquine macular toxicity. Hydroxychloroquine (Plaquenil, Sanofi) is one of the oral therapies used for the treatment of systemic lupus erythematosus and other autoimmune diseases.1-10 Signs of hydroxychloroquine ophthalmic toxicity includes corneal verticilatta, lens opacities and irreversible macular pigmentary damage producing permanent progressive photoreceptor loss with visual alterations.1-10
Retinal pigment epithelium (RPE) melanin binding leads to inhibited RPE lysosome activity and reduced phagocytosis of shed receptor segments.1-5 Accumulation of debris produces photoreceptor atrophy.1-6 Early paramacular RPE pathology can be detected with OCT and fundus autofluorescence imaging before losses create symptoms.6-10Advanced maculo-retinal toxicity produces the classic finding of “bull’s eye” maculopathy with RPE atrophy and impairment.
Pericentral maculopathy without parafoveal (bull’s eye) changes has been documented to occur in Asian populations.1,3,7 Today, testing is required so that clinicians may obtain a wide-angle assay of the fundus for analysis.1,3,7
New information suggests that daily doing exceeding 5mg/kg body weight increases the odds of retinal pathology by 5.7 times. Patients who have taken the preparation continuously for over 10 years increase the risk of retinopathy by 3.2 times.1-5,7 The relative risk increases to 20% when the medications is used for over 20 continuous years, reinforcing the importance of dose-correct prescribing for each individual.1-7
In all cases where rheumatology has committed the patient to this therapy, the ophthalmic literature recommends yearly examination with dilated fundus evaluation. Screening procedures includes OCT with automated field testing regardless of symptom status.6,8-10 Fundus autofluorescence and multifocal electroretinogram may be included.1,5,6,8,9
The American Academy of Ophthalmology recognizes greatest risk in the following scenarios:
(1) When the total amount of medication use exceeds 1,000 total grams (400mg/day x 365 days/yr x 7 yrs. = 1,022,000mg or 1,022g)
(2) When the dosage exceeds 5mg/kg body weight. Example: In a 150 lb. (68kg) person, a limit of 5mg/kg would put them at a 340mg/day maximum dosage, meaning that if this person were taking 2 x 200mg tablets per day they would in theory be taking over the recommended daily dosage.
The literature does document that incidence of toxicity is rare even after exceeding this threshold. However, since damage is both irreversible and progressive (the RPE sequesters the chemicals that produce the damage and their half-life is long), the papers recommend testing biannually for all patients who are either approaching the limit or are over it.1-10
When it is realized that the maximum total dosage is on the horizon or if an adverse event is discovered upon routine or symptomatic testing, suggestions to the medical team should include reducing dosages or choosing alternative agents.1-10
The Bottom Line
Ophthalmic hydroxychloroquine toxicity (corneal/retinal) is clearly related to daily dosage and duration of use. Patients using the medication should obtain a baseline examination at the start of therapy with ancillary testing to identify toxicity over time. Retinal toxicity can produce permanent and progressive visual disability. Management with internal medicine/rheumatology may lead to better dosing, monitoring and alternative choices in high risk cases. Appropriate referral to a retina specialist can prevent damage before it occurs.
This patient was referred to a retina specialist who concurred with the diagnosis and recommended observation/management with routine dilated eye exams and supported our correspondence to the medical “players” to either reduce dosages or change pharmaceuticals. In the end, the daily dosage was lowered.
Dr. Gurwood thanks Karan Johal, OD, for contributing to this case.
Dr. Gurwood is a professor of clinical sciences at The Eye Institute of the Pennsylvania College of Optometry at Salus University. He is a co-chief of Primary Care Suite 3. He is attending medical staff in the department of ophthalmology at Albert Einstein Medical Center, Philadelphia. He has no financial interests to disclose.
|
1. Stokkermans TJ, Goyal A, Trichonas G. Chloroquine and hydroxychloroquine toxicity. In: StatPearls. Treasure Island (FL): StatPearls Publishing; April 17, 2023. 2. Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231-269. 3. Yusuf IH, Charbel Issa P, Ahn SJ. Hydroxychloroquine-induced retinal toxicity. Front Pharmacol. 2023;14(5):1196783. 4. Dima A, Jurcut C, Chasset F, Felten R, Arnaud L. Hydroxychloroquine in systemic lupus erythematosus: overview of current knowledge. Ther Adv Musculoskelet Dis. 2022;14(2):1759720X211073001. 5. Basta F, Fasola F, Triantafyllias K, Schwarting A. Systemic lupus erythematosus (SLE) Therapy: The Old and the New. Rheumatol Ther. 2020;7(3):433-446. 6. Cheong KX, Ong CJT, Chandrasekaran PR, Zhao J, Teo KYC, Mathur R. Review of retinal imaging modalities for hydroxychloroquine retinopathy. Diagnostics (Basel). 2023;13(10):1752. 7. Hsu ST, Ponugoti A, Deaner JD, Vajzovic L. Update on retinal drug toxicities. Curr Ophthalmol Rep. 2021;9(4):168-177. 8. Durán-Carrasco OE, Rodríguez-Gil R, Pérez-Llombet-Quintana N, et al. Microperimetry in hydroxychloroquine macular toxicity. Rom J Ophthalmol. 2021;65(3):235-240. 9. Parrulli S, Cozzi M, Airaldi M, et al. Quantitative autofluorescence findings in patients undergoing hydroxychloroquine treatment. Clin Exp Ophthalmol. 2022;50(5):500-509. 10. Borrelli E, Battista M, Cascavilla ML, et al. Impact of structural changes on multifocal electroretinography in patients with use of hydroxychloroquine. Invest Ophthalmol Vis Sci. 2021;62(12):28. |

