 |
Q:
What are the differences between epi-on and -off CXL and how are protocols being devised to ensure comparable efficacy with the newer procedure?
The paradigm of progressive keratoconus management has shifted tremendously with FDA approval of epithelium-off collagen corneal crosslinking (CXL) in 2016. Previously, we managed patients with an arsenal of optical devices for visual rehabilitation. However, nothing could prevent patients from disease progression—leading to a loss of their best-corrected visual acuity, corneal scarring, contact lens intolerance and inevitably a corneal transplant, Drs. Lawrence Nguyen, OD, and Mitch Ibach, OD, of Vance Thompson Vision in Sioux Falls, SD explain. Now, epi-off CXL can halt the progression of corneal ectasia. The epithelium is debrided to allow better diffusion of the riboflavin into the stroma.1 The riboflavin is then coupled with UV light to stabilize the biomechanically weakened ectatic cornea.
Off’s Limits
Dr. Nguyen emphasizes that with epi-off CXL, post-op care is crucial, especially pain management. “Our first line is to ensure the bandage contact lens is in place, then to advise frequent lubrication and oral NSAIDs,” she says. Reassuring patients that their decreased acuity is normal is also important, she adds. Unlike in laser refractive surgeries, CXL patients are not expected to have a profound visual recovery; it is simply not the goal of this procedure. However, studies have shown improvement of uncorrected visual acuity up to 2.7 Snellen lines after two years.2
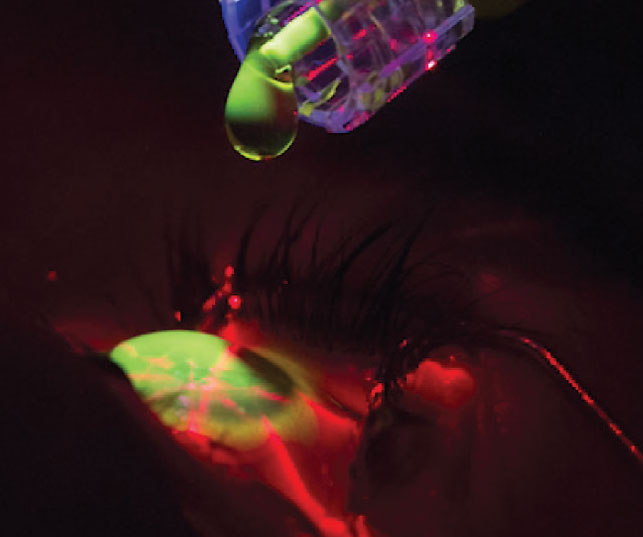 |
|
Riboflavin loading in CXL. Click image to enlarge. |
There is no question that epi-off is efficacious at halting the progression of corneal ectasia. The Avedro Phase III clinical trials demonstrated 1.6D of flattening in Kmax after one year compared to the sham group, which steepened in Kmax.3 Other studies have shown similar outcomes in Kmax flattening.2,4 From a safety standpoint, epi-off CXL is minimally invasive with few risks, including temporary corneal haze, punctate keratitis, corneal scarring/infiltrates and infectious keratitis.3
On the Radar
Now with epithelium-on CXL on the horizon, the goal is to lessen postoperative pain, shorten procedural duration and significantly reduce the risk of corneal haze and infections, Dr. Ibach notes. “Epi-on CXL emphasizes the patient’s quality of life and can allow the patient to return to their normal routine postoperatively at a much faster rate. From a surgical standpoint, this can also shorten the duration of the procedure as well.”
The biggest concern remains whether epi-on is as efficacious compared to epi-off CXL, Dr. Ibach warns. Oxygen, riboflavin and UV light are all necessary for CXL. The intact epithelium adds a barrier that poses a threat to efficacy. Despite epi-off providing superior results in most studies, epi-on CXL has still shown great potential to halt progression through multiple platforms.5,6
Glaukos’s epi-on CXL is currently in an FDA Phase III trial and met the primary endpoint of a 1.00D difference in Kmax between the treatment group and the control group at six months.7 To circumvent the epithelium, there are many variations to epi-on CXL to increase its effectiveness. Some strategies include pulsed UV light treatments, riboflavin loading sponges and specialized goggles to increase oxygen levels. Others are aimed at increasing riboflavin penetration through various formulations, including the addition of sodium iodine or norepinephrine.8,9
“Regardless, CXL has become a valuable and life-changing procedure for patients. It will be exciting to see epi-on available in the US, as each technique will still have its situational advantages in progressive keratoconus patients,” Dr. Ibach posits.
Dr. Shovlin, a senior optometrist at Northeastern Eye Institute in Scranton, PA, is a fellow and past president of the American Academy of Optometry and a clinical editor of Review of Optometry and Review of Cornea & Contact Lenses. He consults for Kala, Aerie, AbbVie, Novartis, Hubble and Bausch + Lomb and is on the medical advisory panel for Lentechs.1. Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34(5):796-801. 2. Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen crosslinking for keratoconus in Italy: the Siena Eye Cross Study. Am J Ophthalmol. 2010;149(4):585-93. 3. Hersh PS, Stulting RD, Muller D, et al. United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment. Ophthalmology. 2017;124(9):1259-70. 4. Wittig-Silva, C, Whiting M, Lamoureux E, et al. A randomized controlled trial of corneal collagen crosslinking in progressive keratoconus: preliminary results. J Refract Surg. 2008;24(7):S720-5. 5. Kobashi H, Rong SS, Ciolino JB. Transepithelial versus epithelium-off corneal crosslinking for corneal ectasia. J Cataract Refract Surg. 2018;44(12):1507-16. 6. Rush SW, Rush RB. Epithelium-off vs. transepithelial corneal collagen crosslinking for progressive corneal ectasia: a randomized and controlled trial. Br J Ophthalmol. 2017;101(4):503-8. 7. Glaukos announces positive Phase III trial results for iLink epi-on investigational therapy [press release]. Eyewire. eyewire.news/articles/glaukos-announces-positive-phase-3-trial-results-for-ilink-epi-on-investigational-therapy/?c4src=article:infinite-scroll. February 26, 2021. Accessed March 10, 2023. 8. Rubinfeld RS, Stulting RD, Gum GG, Talamo JH. Quantitative analysis of corneal stromal riboflavin concentration without epithelial removal. J Cataract Refract Surg. 2018;44(2):237-42. 9. Liu G, Li T, Qi B, et al. Norepinephrine as an enhancer promoting corneal penetration of riboflavin for transepithelial corneal crosslinking. Transl Vis Sci Technol. 2023;12(2):21. |

